
Primary tumor location impacts survival in colorectal cancer patients after primary resection: a population‑based propensity score matching cohort study
Highlight box
Key findings
• Overall survival of right-sided colon cancers (R-CCs) was significantly lower than that of both left-sided colon cancers (L-CCs) and rectal cancer (ReC).
What is known and what is new?
• R-CCs are associated with worse outcomes compared to L-CC, while it is unclear when compared with ReC.
• R-CC and L-CC/ReC are different tumors that have distinct effects on CRC patients with liver metastases.
What is the implication, and what should change now?
• Primary tumor location is an important factor for evaluating survival prognosis of colorectal cancer patients as well as planning the therapeutic schedule.
Introduction
Colorectal cancer (CRC) is a common malignant tumor of the gastrointestinal tract (1) and the second leading cause of cancer-related death (2,3). The difference between left- and right-sided colon cancers (R-CCs) is a focus of CRC research. Multiple researches have confirmed the better overall survival in L-CCs patients, compared with R-CCs (4-6). The difference between the left and right colon was first described in 1990 by Bufill, who proposed that the tumors of each side were different diseases. The right colon, also known as the proximal colon, includes the cecum, ascending colon, hepatic flexure, and transverse colon. The left colon, also known as the distal colon, includes the splenic flexure, descending colon, sigmoid colon, and rectum. Malignant tumors originating in the left and right colon are called left colon cancer and right colon cancer, respectively (7), and the location may account for a significant proportion of the heterogeneity between them. The reason for this discrepancy is the different embryological origins of the left and right colon (the left colon originates from the hindgut and the right colon from the midgut) (8). With changes in dietary and lifestyle habits, the incidence of CRC is rising year by year in China. The main cause of death in CRC patients is distant metastasis, with the liver being the main target organ. About 50% of patients will have liver metastasis, but surgical resection of metastases is possible in only about 10–20% of these patients (9). At present, for patients with unresectable liver metastases, treatment of the primary foci is controversial. Many scholars prefer surgical resection to improve patient prognosis (10), while others hold the opposite view (11). Moreover, the primary tumor location (PLT) may also affect the choice of chemotherapy strategy (12). This study analyzed the role of primary site resection in the management of unresectable liver metastases based on extensive evidence-based medical data from the Surveillance, Epidemiology, and End Results (SEER) database of the National Cancer Institute. The clinical value of resection of primary foci in patients with unresectable liver metastases and its prognostic influences were analyzed. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-71/rc).
Methods
Patient selection
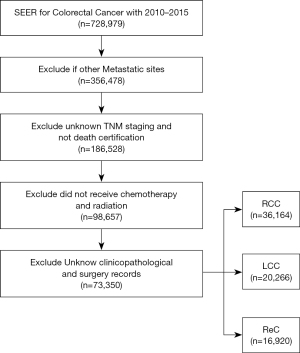
The correlation between PTL and CRC outcomes was the focus of this population-based cohort study. CRC patient data was sourced from the SEER database (SEER*Stat Software version 8.4.0), which covers 27.8% of cancer patients in the United States (13). We followed data and project guidance provided by the North American Association of Central Cancer Registries (NAACCR) to obtain and describe the data (14). Based on the year of diagnosis (NAACCR Item 390), data of all CRC cases from 2010 to 2015 were extracted. Exclusion was based on the criteria of NAACCR Items 490, 2180, and 380. The selection process is shown in Figure 1. Patients with adenocarcinoma were further identified by the International Classification of Diseases for Oncology, Third Edition (ICDO-3) histology codes 8140, 8144, 8210, 8211, 8220, 8221, 8255, 8260, 8261, 8262, and 8263; mucinous 8480; mucin-producing adenocarcinoma 8481; and signet ring cell carcinoma 8490 (NAACCR Item 522). The patients were classified into stages based on NAACCR Item 3000 (DERIVED AJCC-6 STAGE GRP). Right-sided CRC and left-sided CRC were distinguished using NAACCR Items 522 and 523. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Classification
Primary cancer site and histology were identified according to ICDO-3 (15). Right-sided CRC (R-CC) included tumor sites in the transverse colon, cecum, ascending, and hepatic flexure (16). Splenic flexure, descending colon, sigmoid, and rectosigmoid junction tumor sites were considered left-sided CRC (L-CC). Rectal cancer (ReC) included tumors in the rectum.
Statistical analysis
The R statistical software (www.r-project.org) was applied to analyze data. Statistically significant difference was defined a two-sided P value <0.05. Overall survivals (OSs) were the coprimary endpoints. Likelihood-ratio tests were applied to calculate P values. The proportional hazard assumption for Cox regression was tested by scaled Schoenfeld residuals and by inspection of the hazard ratio (HR) plots. The imbalances regarding prognostic factors of patients with different PTL were estimated by multivariable logistic regression with adjustment for age, sex, year of diagnosis, histologic type, grade, American Joint Committee on Cancer (AJCC) 6th edition staging, tumor (T) stage, node (N) stage, metastasis (M) stage, metastatic site (liver or other), marital status, radiation recode, chemotherapy recode (17), lymph node ratio (LNR), and carcinoembryonic antigen (CEA) level. The impact of different PTLs on survival was further assessed with inverse propensity weight adjustment (stabilized weights) using the “ipw” package in R (18). Subsequently, based on the “PSweight” R package, propensity score and weighted analysis of exact matching was performed (19,20). Finally, in order to assess the impact of metastatic resection, a near–far matching analysis was performed, and the unobserved confounding variables were adjusted. These 2 groups were then matched and analyzed in a paired Cox-regression model.
Results
Demographics and tumor characteristics
Of the 73,350 patients who met the inclusion criteria, 36,164 (49.3%) were R-CC, 20,266 (27.6%) were L-CC, and 16, 920 (23.1%) were ReC. As shown in Table 1, there were significant differences in gender, age, tumor grade, tumor location, tumor size, marital status, T stage, N stage, CEA, and other factors among the patients enrolled in the study (P<0.001). Gender, age, and marital status were significantly different between groups (P<0.001). The ReC group included the highest percentage of male patients (59.2%), whereas the R-CC group had the highest percentage of female patients (53%). The R-CC group had the most patients ≥60. There was a significant difference in marital status among the 3 groups, and the proportion of L-CC tumors with a better prognosis was higher in patients with stable marital status (Table 1). Only the variables of year of diagnosis and combined with liver metastases showed no significant difference (Table 1, P>0.05).
Table 1
| Clinicopathological variables | Unweighted result, n (%) | PSM result, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| R-CC | L-CC | ReC | P | R-CC | L-CC | ReC | P | ||
| Total | 36,164 (49.3) | 20,266 (27.6) | 16,920 (23.1) | 8,670.00 | 8,670.00 | 8,670.00 | |||
| Age, years | <0.001 | 0.25 | |||||||
| <60 | 8,017 (22.2) | 7,720 (38.1) | 7,527 (44.5) | 1,612 (18.6) | 3,624 (41.8) | 4,136 (47.7) | |||
| ≥60 | 28,147 (77.8) | 12,546 (61.9) | 9,393 (55.5) | 7,058 (81.4) | 5,046 (58.2) | 4,534 (52.3) | |||
| Sex | <0.001 | 0.18 | |||||||
| Male | 16,986 (47.0) | 11,029 (54.4) | 10,025 (59.2) | 4,188 (48.3) | 4,517 (52.1) | 5,089 (58.7) | |||
| Female | 19,178 (53.0) | 9,237 (45.6) | 6,895 (40.8) | 4,482 (51.7) | 4,153 (47.9) | 3,581 (41.3) | |||
| Year of diagnosis | 0.11 | 0.96 | |||||||
| 2010–2012 | 17,818 (49.3) | 10,209 (50.4) | 8,257 (48.8) | 4,370 (50.4) | 4,439 (51.2) | 4,309 (49.7) | |||
| 2013–2015 | 18,346 (50.7) | 10,057 (49.6) | 8,663 (51.2) | 4,300 (49.6) | 4,231 (48.8) | 4,361 (50.3) | |||
| Histologic type | <0.001 | <0.001 | |||||||
| Adenocarcinomas | 30,965 (85.6) | 18,806 (92.8) | 15,875 (93.8) | 7,213 (83.2) | 7,864 (90.7) | 7,985 (92.1) | |||
| Non-adenocarinomas | 5,199 (14.4) | 1,460 (7.2) | 1,045 (6.2) | 1,457 (16.8) | 806 (9.3) | 685 (7.9) | |||
| Grade | <0.001 | 0.08 | |||||||
| Grade I | 2,463 (6.8) | 1,489 (7.3) | 1,124 (6.6) | 511 (5.9) | 599 (6.9) | 546 (6.3) | |||
| Grade II | 24,373 (67.4) | 15,682 (77.4) | 13,362 (79.0) | 6,008 (69.3) | 6,658 (76.8) | 6,745 (77.8) | |||
| Grade III | 7,590 (21.0) | 2,605 (12.9) | 2,089 (12.3) | 2,037 (23.5) | 980 (11.3) | 997 (11.5) | |||
| Grade IV | 1,738 (4.8) | 490 (2.4) | 345 (2.0) | 114 (1.3) | 433 (5.0) | 382 (4.4) | |||
| AJCC 6th ed | <0.001 | 0.07 | |||||||
| I | 6,698 (18.5) | 3,558 (17.6) | 3,136 (18.5) | 1,518 (17.5) | 1,326 (15.3) | 1,491 (17.2) | |||
| II | 12,555 (34.7) | 6,102 (30.1) | 4,652 (27.5) | 3,190 (36.8) | 2,462 (28.4) | 2,124 (24.5) | |||
| III | 11,530 (31.9) | 7,154 (35.3) | 7,188 (42.5) | 2,662 (30.7) | 3,286 (37.9) | 3,867 (44.6) | |||
| IV | 5,381 (14.9) | 3,452 (17.0) | 1,944 (11.5) | 1,300 (15.0) | 1,596 (18.4) | 1,188 (13.7) | |||
| T-stage | <0.001 | <0.001 | |||||||
| T1 | 2,699 (7.5) | 1,846 (9.1) | 1,476 (8.7) | 598 (6.9) | 988 (11.4) | 590 (6.8) | |||
| T2 | 5,424 (15.0) | 2,769 (13.7) | 2,861 (16.9) | 1,231 (14.2) | 1,379 (15.9) | 1,743 (20.1) | |||
| T3 | 20,827 (57.6) | 11,646 (57.5) | 10,514 (62.1) | 5,531 (63.8) | 4,681 (54.0) | 5,020 (57.9) | |||
| T4 | 7,214 (19.9) | 4,005 (19.8) | 2,069 (12.2) | 1,310 (15.1) | 1,622 (18.7) | 1,317 (15.2) | |||
| N-stage | <0.001 | <0.001 | |||||||
| N0 | 20,085 (55.5) | 10,346 (51.1) | 8,168 (48.3) | 5,124 (59.1) | 4,170 (48.1) | 4,578 (52.8) | |||
| N1 | 9,200 (25.4) | 6,196 (30.6) | 5,771 (34.1) | 1,726 (19.9) | 2,818 (32.5) | 2,592 (29.9) | |||
| N2 | 6,879 (19.0) | 3,724 (18.4) | 2,981 (17.6) | 1,820 (21.0) | 1,682 (19.4) | 1,500 (17.3) | |||
| M-stage | <0.001 | <0.001 | |||||||
| M0 | 30,783 (85.1) | 16,814 (83.0) | 14,976 (88.5) | 7,638 (88.1) | 7,023 (81.0) | 7,725 (89.1) | |||
| M1 | 5,381 (14.9) | 3,452 (17.0) | 1,944 (11.5) | 1,032 (11.9) | 1,647 (19.0) | 945 (10.9) | |||
| Combined diagnosis-liver | 0.34 | 0.05 | |||||||
| Yes | 3,553 (9.8) | 2,591 (12.8) | 1,393 (8.2) | 754 (8.7) | 1,162 (13.4) | 806 (9.3) | |||
| No | 32,611 (90.2) | 17,675 (87.2) | 15,527 (91.8) | 7,916 (91.3) | 7,508 (86.6) | 7,864 (90.7) | |||
| Surg Oth Reg/Dis | <0.001 | 0.75 | |||||||
| Yes | 2,844 (7.9) | 1,812 (8.9) | 1,352 (8.0) | 533 (6.2) | 660 (7.7) | 815 (9.4) | |||
| No | 33,302 (92.1) | 18,444 (91.0) | 15,557 (91.9) | 8,132 (93.8) | 8,002 (92.3) | 7,846 (90.6) | |||
| Unknown | 18 (0.0) | 10 (0.0) | 11 (0.1) | 5 (0.0) | 8 (0.1) | 9 (0.1) | |||
| Primary tumor size | <0.001 | 0.69 | |||||||
| <5 cm | 19,276 (53.3) | 12,369 (61.0) | 10,089 (59.6) | 4,552 (52.5) | 5,497 (63.4) | 5,297 (61.1) | |||
| ≥5 cm | 16,888 (46.7) | 7,897 (39.0) | 6,831 (40.4) | 4,118 (47.5) | 3,173 (36.6) | 3,373 (38.9) | |||
| Marital status | <0.001 | 0.04 | |||||||
| Married | 19,787 (54.7) | 11,711 (57.8) | 10,149 (60.0) | 4,500 (51.9) | 5,115 (59.0) | 5,367 (61.9) | |||
| Single | 5,117 (14.1) | 3,641 (18.0) | 2,897 (17.1) | 1,361 (15.7) | 1,587 (18.3) | 1,439 (16.6) | |||
| SDW | 11,260 (31.1) | 4,914 (24.2) | 3,874 (22.9) | 2,809 (32.4) | 1,968 (22.7) | 1,864 (21.5) | |||
| Radiation recode | <0.001 | 0.08 | |||||||
| Yes | 470 (1.3) | 624 (3.1) | 8,771 (51.8) | 78 (0.9) | 303 (3.5) | 4,430 (51.1) | |||
| No | 35,964 (98.7) | 19,642 (96.9) | 8,149 (48.2) | 8,592 (99.1) | 8,367 (96.5) | 4,240 (48.9) | |||
| Chemotherapy recode | <0.001 | 0.40 | |||||||
| Yes | 13,081 (36.2) | 9,268 (45.7) | 11,339 (67.0) | 3,026 (34.9) | 3,890 (44.9) | 5,982 (69.0) | |||
| No | 23,083 (63.8) | 10,998 (54.3) | 5,581 (33.0) | 5,644 (65.1) | 4,777 (55.1) | 2,688 (31.0) | |||
| LNR | <0.001 | <0.001 | |||||||
| <0.3 | 31,442 (86.9) | 17,557 (86.6) | 14,960 (88.4) | 7,560 (87.2) | 7,655 (88.3) | 7,716 (89.0) | |||
| ≥0.3 | 4,722 (13.1) | 2,709 (13.4) | 1,960 (11.6) | 1,110 (12.8) | 1,015 (11.7) | 954 (11.0) | |||
| CEA level | <0.001 | 0.13 | |||||||
| Positive | 15,041 (41.6) | 8,932 (44.1) | 7,275 (43.0) | 3,546 (40.9) | 4,023 (46.4) | 3,884 (44.8) | |||
| Negative | 21,123 (58.4) | 11,334 (55.9) | 9,645 (57.0) | 5,124 (59.1) | 4,647 (53.6) | 4,786 (55.2) | |||
The data in brackets represent the number of this item accounts for the total number of clinicopathological variables in a kind of colorectal cancer. For example, 22.2% is the number of patients whose age <60 (8,017) accounts for total number of R-CC (36,164). PSM, propensity score matching; R-CC, right-sided colon cancer; L-CC, left-sided colon cancer; ReC, rectal cancer; AJCC, American Joint Committee on Cancer; Combined diagnosis -liver, with liver metastasis; Surg Oth Reg/Dis, having surgical operation on metastatic foci; SDW, separated, divorced and widowed; LNR, lymph node ratio; CEA, carcinoembryonic antigen.
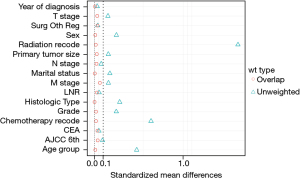
After propensity score matching (PSM) was used to balance the differences in baseline characteristics among the 3 groups, 8,670 patients in each group were screened out by matching in a ratio of 1:1. Analysis of the characteristics of the 3 groups of cases after matching showed that the propensity score distribution of the 3 groups of cases was consistent. The standardized mean difference (SMD) was significantly reduced after propensity score weighting (SMD <0.1), and the matching score was stable and reliable (Figure 2). This indicated that the case data of the 3 groups had better comparability after matching. On this basis, the differences after matching were compared, and the results showed that the differences were significantly reduced (Table 1). Gender, marital status, histological type, TNM stage, liver metastasis, LNR, and CEA were statistically significant before and after PSM. There was no difference in whether the metastases were treated surgically among the 3 groups (P=0.896). At the end of follow-up, 43,405 (59.2%) patients were alive. Overall, 7,537 (10.3%) patients had liver metastases only, and 10,777 (14.7%) patients had distant metastases.
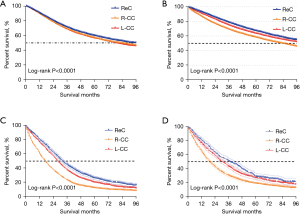
OS and liver metastasis
We then analyzed the OS before and after PSM. R-CC patients were associated with worse prognosis when compared to L-CC and ReC patients (Figure 3). The median OS of R-CC before and after PSM was 76.5 vs. 84.2 months (P<0.001), in L-CC it was 79.3 vs. 102.1 months (P<0.001), and in ReC it was 91.7 vs. 104.9 months (P<0.001). The results showed the relationship between OS and primary tumor in patients with liver metastases was clearer, and the stratification was clearer.
In the survival analysis of the subgroup with liver metastases and the subgroup with metastases from other sites, the median OS of R-CC, L-CC, and ReC were 17 vs. 27 months (P<0.001), 28 vs. 37 months (P<0.001), and 32 vs. 43 months (P<0.001), respectively.
In summary, the OS rate in R-CC patients was significantly lower than those in the L-CC and ReC groups after PSM and in the Kaplan–Meier curve of the liver metastasis subgroup.
Survival benefits of clinical factors
After the PSM adjustment, multivariable Cox regression analysis was used to examine the risk factors associated with CRC mortality (Table 2). The risk factors of mortality were age ≥60 years old [vs. <60 years old; HR, 0.563; 95% confidence index (CI), 0.547–0.580; P=0.0438]; being separated, divorced, or widowed (SDW) (vs. single; HR, 0.719; 95% CI: 0.700–0.739; P=0.039); L-CC (vs. R-CC; HR, 1.113; 95% CI: 1.071–1.158; P<0.001) and ReC (vs. R-CC; HR, 1.166; 95% CI: 1.134–1.199; P<0.001); non-adenocarcinomas (vs. adenocarcinomas; HR, 1.126; 95% CI: 1.087–1.166; P<0.001); T2 (vs. T1; HR, 0.375; 95% CI: 0.344–0.408; P<0.001), T3 (vs. T1; HR, 0.456; 95% CI: 0.426–0.488; P<0.001), and T4 (vs. T1; HR, 0.635; 95% CI: 0.618–0.653; P<0.001); N1 (vs. N0; HR, 0.722; 95% CI: 0.674–0.773; P<0.001) and N2 (vs. N0; HR, 0.873; 95% CI: 0.839–0.908; P<0.001); combined diagnosis (DX)-liver (vs. no combined DX-liver; HR, 0.735; 95% CI: 0.701–0.771; P<0.001); chemotherapy recode (vs. no chemotherapy recode; HR, 2.050; 95% CI: 1.991–2.111; P<0.001); LNR ≥0.3 (vs. LNR <0.3; HR, 0.634; 95% CI: 0.609–0.661; P<0.001); and CEA level positive (vs. CEA level negative; HR, 0.677; 95% CI: 0.637–0.694; P<0.001).
Table 2
| Variables | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age, years | |||||||
| <60 | Ref | ||||||
| ≥60 | 0.578 | 0.563–0.594 | <0.001 | 0.563 | 0.547–0.580 | 0.0438 | |
| Sex | |||||||
| Female | Ref | ||||||
| Male | 0.946 | 0.924–0.967 | <0.001 | 0.829 | 0.810–0.849 | 0.221 | |
| Marital status | |||||||
| Single | Ref | ||||||
| Married | 0.805 | 0.778–0.833 | <0.001 | 0.894 | 0.863–0.926 | 0.065 | |
| SDW | 0.656 | 0.639–0.673 | <0.001 | 0.719 | 0.700–0.739 | 0.039 | |
| Primary site | |||||||
| R-CC | Ref | ||||||
| L-CC | 0.884 | 0.855–0.914 | <0.001 | 1.113 | 1.071–1.158 | <0.001 | |
| ReC | 1.224 | 1.191–1.257 | <0.001 | 1.166 | 1.134–1.199 | <0.001 | |
| Primary tumor size | |||||||
| <5 cm | Ref | ||||||
| ≥5 cm | 0.708 | 0.692–0.724 | <0.001 | 0.983 | 0.960–1.007 | 0.163 | |
| Year of diagnosis | |||||||
| 2010–2012 | Ref | ||||||
| 2013–2015 | 1.068 | 1.043–1.094 | 0.231 | 1.038 | 1.013–1.063 | 0.137 | |
| Histologic type | |||||||
| Adenocarcinomas | Ref | ||||||
| Unadenocarinomas | 1.431 | 1.384–1.480 | <0.001 | 1.126 | 1.087–1.166 | <0.001 | |
| Grade (thru 2017) | |||||||
| Grade I | Ref | ||||||
| Grade II | 0.418 | 0.390–0.449 | <0.001 | 0.687 | 0.639–0.738 | 0.067 | |
| Grade III | 0.516 | 0.489–0.544 | <0.001 | 0.727 | 0.689–0.768 | 0.237 | |
| Grade IV | 0.844 | 0.797–0.893 | <0.001 | 0.892 | 0.843–0.945 | 0.148 | |
| AJCC 6th ed | |||||||
| I | Ref | ||||||
| II | 0.145 | 0.139–0.151 | 0.501 | 0.297 | 0.270–0.327 | 0.201 | |
| III | 0.215 | 0.209–0.222 | 0.161 | 0.273 | 0.255–0.293 | 0.176 | |
| IV | 0.291 | 0.283–0.300 | 0.061 | 0.389 | 0.370–0.408 | 0.067 | |
| T-stage | |||||||
| T1 | Ref | ||||||
| T2 | 0.208 | 0.195–0.220 | <0.001 | 0.375 | 0.344–0.408 | <0.001 | |
| T3 | 0.28 | 0.268–0.292 | <0.001 | 0.456 | 0.426–0.488 | <0.001 | |
| T4 | 0.469 | 0.457–0.481 | <0.001 | 0.635 | 0.618–0.653 | <0.001 | |
| N-stage | |||||||
| N0 | Ref | ||||||
| N1 | 0.334 | 0.325–0.344 | <0.001 | 0.722 | 0.674–0.773 | <0.01 | |
| N2 | 0.52 | 0.505–0.536 | <0.001 | 0.873 | 0.839–0.908 | <0.01 | |
| M-stage | |||||||
| M0 | Ref | ||||||
| M1 | 0.229 | 0.223–0.235 | <0.001 | 0.853 | 0.820–0.897 | <0.001 | |
| Combined diagnosis-liver | |||||||
| No | Ref | ||||||
| Yes | 0.24 | 0.234–0.247 | <0.001 | 0.735 | 0.701–0.771 | <0.001 | |
| Surg Oth Reg/Dis | |||||||
| No | Ref | ||||||
| Yes | 0.967 | 0.583–1.604 | 0.896 | 1.316 | 0.793–2.184 | 0.288 | |
| Unknown | 1.711 | 1.030–2.841 | 0.038 | 1.207 | 0.727–2.005 | 0.467 | |
| Radiation recode | |||||||
| No | Ref | ||||||
| Yes | 0.761 | 0.734–0.788 | 0.201 | 0.905 | 0.865–0.948 | 0.087 | |
| Chemotherapy recode | |||||||
| No | Ref | ||||||
| Yes | 0.976 | 0.954–0.998 | 0.033 | 2.05 | 1.991–2.111 | 0.041 | |
| LNR | |||||||
| <0.3 | Ref | ||||||
| ≥0.3 | 0.321 | 0.312–0.330 | <0.001 | 0.634 | 0.609–0.661 | <0.001 | |
| CEA level | |||||||
| Negative | Ref | ||||||
| Positive | 2.168 | 2.119–2.218 | <0.001 | 0.677 | 0.637–0.694 | <0.001 | |
PSM, propensity score matching; HR, hazard ratio; CI, confidence interval; SDW, separated, divorced and widowed; R-CC, right-sided colon cancer; L-CC, left-sided colon cancer; ReC, rectal cancer; AJCC, American Joint Committee on Cancer; Surg Oth Reg/Dis, having surgical operation on metastatic foci; LNR, lymph node ratio; CEA, carcinoembryonic antigen.
In multivariate Cox hazard regression for OS in the liver metastases subgroup, PTL factors had a stronger association with OS than other factors (HR, 1.344, P<0.001) (Table 3).
Table 3
| Primary site | HR | 95% CI | P value |
|---|---|---|---|
| Age, years | |||
| <60 | Ref | ||
| ≥60 | 1.259 | 1.193–1.330 | <0.001 |
| Sex | |||
| Female | Ref | ||
| Male | 1.031 | 0.979–1.086 | 0.244 |
| Marital status | |||
| Single | Ref | ||
| Married | 0.862 | 0.806–0.922 | <0.001 |
| SDW | 1.016 | 0.939–1.098 | 0.698 |
| Year of diagnosis | |||
| 2010–2012 | Ref | ||
| 2013–2015 | 0.961 | 0.913–1.012 | 0.129 |
| Primary site | |||
| R-CC | Ref | ||
| L-CC | 1.344 | 1.249–1.446 | <0.001 |
| ReC | 1.244 | 1.168–1.326 | <0.001 |
| Histologic type | |||
| Adenocarcinomas | Ref | ||
| Non-adenocarinomas | 0.879 | 0.806–0.959 | 0.004 |
| Grade (THRU 2017) | |||
| Grade I | Ref | ||
| Grade II | 0.99 | 0.862–1.136 | 0.881 |
| Grade III | 1.302 | 1.127–1.505 | <0.001 |
| Grade IV | 1.568 | 1.319–1.865 | <0.001 |
| AJCC 6th ed | |||
| I | Ref | ||
| II | 0.382 | 0.351–0.502 | <0.001 |
| III | 0.351 | 0.328–0.468 | <0.001 |
| IV | 0.471 | 0.439–0.499 | <0.001 |
| T-stage | |||
| T1 | Ref | ||
| T2 | 0.838 | 0.617–1.137 | 0.255 |
| T3 | 1.087 | 0.836–1.414 | 0.532 |
| T4 | 1.453 | 1.115–1.892 | 0.006 |
| N-stage | |||
| N0 | Ref | ||
| N1 | 1.285 | 1.188–1.390 | <0.001 |
| N2 | 1.329 | 1.212–1.458 | <0.001 |
| M-stage | |||
| M0 | Ref | ||
| M1 | 0.927 | 0.894–1.221 | <0.001 |
| Surg Oth Reg/Dis | |||
| Yes | Ref | ||
| No | 0.75 | 0.709–0.794 | <0.001 |
| Unknown | 0.617 | 0.293–1.299 | 0.203 |
| Primary tumor size | |||
| <5 cm | Ref | ||
| ≥5 cm | 1.1 | 1.045–1.157 | <0.001 |
| Radiation recode | |||
| Yes | Ref | ||
| No | 1.114 | 1.005–1.236 | 0.41 |
| Chemotherapy recode | |||
| Yes | Ref | ||
| No | 0.416 | 0.390–0.440 | <0.001 |
| LNR | |||
| <0.3 | Ref | ||
| ≥0.3 | 1.436 | 1.338–1.541 | <0.001 |
| CEA level | |||
| Positive | Ref | ||
| Negative | 1.546 | 1.444–1.655 | <0.001 |
HR, hazard ratio; CI, confidence interval; SMD, standardized mean difference; R-CC, right-sided colon cancer; L-CC, left-sided colon cancer; ReC, rectal cancer; AJCC, American Joint Committee on Cancer; Surg Oth Reg/Dis, having surgical operation on metastatic foci; LNR, lymph node ratio; CEA, carcinoembryonic antigen.
Thus, CRC and CRC with liver metastases subgroup patients shared several favorable prognostic factors (age <60, married, low LNR, and low TNM stage) and a common unfavorable prognostic factor (R-CC).
Discussion
The present study reported the first population-based analysis of propensity score adjustments to investigate the prognostic impact of PTL in metastatic colorectal cancer (mCRC) patients with primary tumor removal. This study adjusted for strong bias in various patient and tumor characteristics by using PSM, and the results found that the prognosis of patients with liver metastasis from left colon cancer and ReC was better than that of patients with right colon cancer. This conclusion was also true in the subgroup of colon cancer patients with liver metastases, and the correlation was even more significant.
Left and right colon cancers have different genetic signatures, so the location of the primary tumor should be considered when choosing chemotherapy or immunotherapy regimens for advanced CRC (21,22). A retrospective study of stage IV CRC patients who underwent liver metastases resection found that patients with R-CC had a higher probability of KRAS mutations and worse survival. In group analysis, however, tumor location was not associated with survival (23). Genomic analysis of patients with metastatic CRC showed the characteristic mutation frequency of left CRC was significantly different from that of right CRC, and in a multivariate model that considered all significant mutations, OS was independent of the location of the primary tumor (24,25). The above results demonstrated the importance of gene mutation differences on the outcome of patients with metastatic disease, while the primary location of the tumor is lack of in-depth study.
Multiple studies have suggested that the PTL influenced the prognosis of patients with advanced CRC, and patients with left-sided colon cancer (L-CC) had a better OS compared with that of patients with R-CCs (26-28). However, whether PTL was associated with long-term survival in patients with CRC liver metastases after radical resection of the primary tumor and metastatic tumor remains inconclusive. Our study used data of patients in the SEER database for survival analysis. The SEER database contains a large sample size and is used by the majority of clinical researchers. PSM reduces the effect of individual confounding factors and selective propensity on study results and is a method that can simultaneously match multiple variables to balance baseline differences (29,30). In the present study, we used the PSM method to investigate the effect of primary tumor resection of CRC on the prognosis and survival of colorectal liver metastases (CRLM) patients with unresectable metastases using a large amount of real clinical data from the SEER database. The results revealed that the OS of the left liver metastasis group and the nonmetastasis group was significantly longer than that in the right tumor group before and after matching, indicating that primary tumor resection had obvious advantages in the long-term survival and prognosis of patients. In the analysis of the general data of the patients, it was found that most of the patients whose primary tumor was located in the right colon were in a relatively older age group, with later T and N stages, and poorer differentiation by pathology.
In addition, the influence of primary tumor side of colon cancer on liver metastasis was analyzed. The results of multivariable analysis suggested that OS in patients with right-sided tumors was significantly reduced after recurrence. Therefore, the location of primary tumor is a factor worth considering when deciding on treatment strategies.
The location of the primary tumor was not included in previous risk assessments of CRC liver metastases recurrence. In the analysis of independent prognostic factors for patients, the influence of primary tumor site on the prognosis of patients is still unclear. Some scholars believe that the PTL has no significant impact on the improvement of long-term survival and prognosis of CRLM patients (31), while others believe that compared with a left primary tumor, the prognosis of patients with a right primary tumor is poor (32,33). The results of this study supported the latter view, with patients with right-sided colonic lesions having a shorter OS than those with left-sided colonic lesions, and patients with rectal primary lesions having the highest prognostic score. Previous study has reported that marital status may have an important impact on the prognosis of CRC patients (34). This study was the first to investigate the impact of marital status on the survival prognosis of CRLM patients, and the results showed that married patients could benefit both in terms of OS and cancer-specific survival (CSS) compared with nonmarried patients. Further, the results of this study also showed that factors such as advanced patient age, advanced N stage, and lack of radiotherapy were independent risk factors for survival and prognosis of CRLM patients.
The incidence of liver metastases from colon cancer is high. Currently, systemic chemotherapy and local surgery are used in clinical treatment of liver metastases from colon cancer. Although liver resection is currently the most effective local control treatment, the 5-year survival rate of patients after hepatectomy is low, and the recurrence rate after surgery is as high as 70%. One point of contention has been the dispute over the significance of the left and right colon for CRC. There are important differences between left and right colon cancers in demographics, clinical features, tumor properties, molecular mechanisms, treatment effects, and survival prognosis.
The poor clinical prognosis of CRLM makes effective treatment of patients challenging. For the treatment of metastases, it is often advocated that simultaneous resection should be used to improve the prognosis of patients. However, for patients with unresectable metastases, the treatment plan is mostly negotiated by a multidisciplinary medical team. There is still no consensus on the management of primary lesions of CRC. Therefore, in the context of current advances in CRLM treatment, studies with large samples of primary tumor resection are of great value.
Several factors affect the survival rate of patients with left or right colon tumors. Firstly, the parts in each side of the colon develop from different sources (35). Secondly, different parts of the colon have different functions, with the right colon mainly absorbing water, while the left colon promotes the passage of intestinal contents. In addition, the composition of intestinal microbiota in the right-sided and left-sided colon differs (36). Fusobacterium nucleatum, which has been reported to promote chemoresistance by modulating autophagy, were found in relatively higher abundance in left-sided CRC (37). Finally, gene mutations vary greatly in right-sided and left-sided tumors (38,39). Studies have reported that KRAS mutation and BRAF V600E mutation are predictive markers of resistance to epidermal growth factor-targeted antibodies (40,41), and BRAF V600E mutation is more common in proximal tumors (42). Furthermore, TP53 mutation frequency is higher in distal tumors (43), and metastatic CRC patients with TP53 mutation have been reported to have shorter survival after receiving chemotherapy (44). These findings indicated that the changes of tumor specific molecules vary with the location of the primary tumor.
Our study proposed that PTL could be used as an additional factor to measure the expected outcome of liver metastases resection. Studies have shown that vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) have important roles in the formation and development of CRC liver metastases, as does the expression of VEGF and EGFR in primary tumors. The increase of β-γ is detrimental to patient prognosis, and surgical resection suppresses its expression (45,46). At the same time, the existence of the primary tumor may also induce the adjacent tissue of the metastatic tumor to generate a tumor microenvironment that is conducive to tumor cell invasion, metastasis, growth, and reproduction, and the presence of tumor stem cells in the primary tumor also increases VEGF expression, thereby inducing angiogenesis and jointly promoting tumor development (47). In addition, the persistence of inflammatory factors in the primary tumor of CRC can also affect the development of tumors. Among them, inflammatory factors interferon gamma (IFN-γ) and interleukin-10 (IL-10) can exert antitumor immunity or induce tumor immune resistance. Tumor development is regulated by other processes (48), and thus resection of the primary CRC may reverse the above-mentioned adverse factors, thereby improving the prognosis of CRLM patients. Further, with the continuous development of modern minimally invasive concepts, various techniques such as laparoscopic-assisted surgery and robot-assisted surgery have gradually matured, improving the surgical resection effect of the primary tumor, reducing adverse reactions, and shortening postoperative recovery time, which allows patients to have a higher quality of life and also enables earlier initiation of systemic chemotherapy, thereby improving patient outcomes (49,50).
This study had some shortcomings. Although the study was based on a large amount of real data from the US SEER database, it was still limited by the existing data. First, this study was only based on patients whose primary tumor had been surgically treated. Second, the SEER database does not provide information on the extent of liver metastases, the type of liver resection, the margin of resection (R0 or R1), postoperative chemotherapy regimen and duration, radiotherapy dose and time, and tumor metastasis time and number. In addition, the SEER database lacks information regarding BRAF mutations as well as microsatellite instability, vascular nerve infiltration, and gene expression, which thus could not be further analyzed. Finally, the SEER database only provides information on OS and not disease progression or the tumor-free survival period, which may have led to bias in the analysis of tumor patients. Although this study used PSM to balance the differences in the baseline characteristics of patients, there were still some parameters that could not be balanced due to the interaction of many factors. Therefore, our research conclusions have certain limitations and as a result, multicenter, large-sample evidence-based studies are needed for further verification in the future.
Conclusions
This population-based propensity score-adjusted analysis of mCRC patients provided compelling evidence that the survival rate of patients with surgically resected liver metastases from CRC varies with the location of the primary tumor. The survival rate of patients with R-CC was significantly lower than that of patients with L-CC or ReC. Whether the patient has an R-CC tumor or L-CC and ReC tumor is an important factor to be considered before surgery for metastatic disease. Targeted treatment strategies based on the location of the primary tumor may improve the prognosis of patients with R-CC.
Acknowledgments
Funding: This study was supported by the Dalian Deng Feng Program: Key medical specialties in construction, funded by the People’s Government of Dalian Municipality (No. 243, 2021).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-71/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-71/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-71/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Biller LH, Schrag D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021;325:669-85. [Crossref] [PubMed]
- Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol 2019;16:713-32. [Crossref] [PubMed]
- Kanth P, Inadomi JM. Screening and prevention of colorectal cancer. BMJ 2021;374:n1855. [Crossref] [PubMed]
- Ramadan M, Alfayea T, Alsofyani A, et al. Primary tumor location and survival among metastatic colorectal cancer patients treated with systemic chemotherapy and biologic therapies: Retrospective analysis. Cancer Treat Res Commun 2022;33:100632. [Crossref] [PubMed]
- Chen TH, Chen WS, Jiang JK, et al. Effect of Primary Tumor Location on Postmetastasectomy Survival in Patients with Colorectal Cancer Liver Metastasis. J Gastrointest Surg 2021;25:650-61. [Crossref] [PubMed]
- Brouwer NPM, van der Kruijssen DEW, Hugen N, et al. The Impact of Primary Tumor Location in Synchronous Metastatic Colorectal Cancer: Differences in Metastatic Sites and Survival. Ann Surg Oncol 2020;27:1580-8. [Crossref] [PubMed]
- Bufill JA. Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med 1990;113:779-88. [Crossref] [PubMed]
- Kwak HD, Ju JK, Lee SY, et al. Comparison of Right-side and Left-side Colon Cancers Following Laparoscopic Radical Lymphadenectomy. J Invest Surg 2021;34:142-7. [Crossref] [PubMed]
- Page AJ, Cosgrove DC, Herman JM, et al. Advances in understanding of colorectal liver metastasis and implications for the clinic. Expert Rev Gastroenterol Hepatol 2015;9:245-59. [Crossref] [PubMed]
- Faron M, Pignon JP, Malka D, et al. Is primary tumour resection associated with survival improvement in patients with colorectal cancer and unresectable synchronous metastases? A pooled analysis of individual data from four randomised trials. Eur J Cancer 2015;51:166-76. [Crossref] [PubMed]
- Boselli C, Renzi C, Gemini A, et al. Surgery in asymptomatic patients with colorectal cancer and unresectable liver metastases: the authors' experience. Onco Targets Ther 2013;6:267-72. [PubMed]
- Tharin Z, Blanc J, Alaoui IC, et al. Influence of first line chemotherapy strategy depending on primary tumor location in metastatic colorectal cancer. J Gastrointest Oncol 2021;12:1509-17. [Crossref] [PubMed]
- Doll KM, Rademaker A, Sosa JA. Practical Guide to Surgical Data Sets: Surveillance, Epidemiology, and End Results (SEER) Database. JAMA Surg 2018;153:588-9. [Crossref] [PubMed]
- Wingo PA, Jamison PM, Hiatt RA, et al. Building the infrastructure for nationwide cancer surveillance and control--a comparison between the National Program of Cancer Registries (NPCR) and the Surveillance, Epidemiology, and End Results (SEER) Program (United States). Cancer Causes Control 2003;14:175-93. [Crossref] [PubMed]
- Tomás CC, Oliveira E, Sousa D, et al. Proceedings of the 3rd IPLeiria's International Health Congress: Leiria, Portugal. 6-7 May 2016. BMC Health Serv Res 2016;16 Suppl 3:200.
- Fritz AG. International classification of diseases for oncology: ICD-O. Third edition, First revision. ed. Geneva: World Health Organization; 2013. viii, 242 pages p.
- Noone AM, Lund JL, Mariotto A, et al. Comparison of SEER Treatment Data With Medicare Claims. Med Care 2016;54:e55-64. [Crossref] [PubMed]
- Zeng S, Li F, Wang R, et al. Propensity score weighting for covariate adjustment in randomized clinical trials. Stat Med 2021;40:842-58. [Crossref] [PubMed]
- Joffe MM, Rosenbaum PR. Invited commentary: propensity scores. Am J Epidemiol 1999;150:327-33. [Crossref] [PubMed]
- Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med 1997;127:757-63. [Crossref] [PubMed]
- Gallois C, Pernot S, Zaanan A, et al. Colorectal Cancer: Why Does Side Matter? Drugs 2018;78:789-98. [Crossref] [PubMed]
- Hu H, Zhang Q, Huang R, et al. Genomic Analysis Reveals Heterogeneity Between Lesions in Synchronous Primary Right-Sided and Left-Sided Colon Cancer. Front Mol Biosci 2021;8:689466. [Crossref] [PubMed]
- Margonis GA, Amini N, Buettner S, et al. The Prognostic Impact of Primary Tumor Site Differs According to the KRAS Mutational Status: A Study By the International Genetic Consortium for Colorectal Liver Metastasis. Ann Surg 2021;273:1165-72. [Crossref] [PubMed]
- Yaeger R, Chatila WK, Lipsyc MD, et al. Clinical Sequencing Defines the Genomic Landscape of Metastatic Colorectal Cancer. Cancer Cell 2018;33:125-136.e3. [Crossref] [PubMed]
- Li N, Zhou Z, Zhang L, et al. High expression of TTC21A predict poor prognosis of colorectal cancer and influence the immune infiltrating level. Transl Cancer Res 2022;11:981-92. [Crossref] [PubMed]
- Zhao B, Lopez NE, Eisenstein S, et al. Synchronous metastatic colon cancer and the importance of primary tumor laterality - A National Cancer Database analysis of right- versus left-sided colon cancer. Am J Surg 2020;220:408-14. [Crossref] [PubMed]
- Kaczirek K. ASCO 2016 - update colorectal liver metastases. Memo 2017;10:103-5. [Crossref] [PubMed]
- Mirón Fernández I, Mera Velasco S, Turiño Luque JD, et al. Right and Left Colorectal Cancer: Differences in Post-Surgical-Care Outcomes and Survival in Elderly Patients. Cancers (Basel) 2021;13:2647. [Crossref] [PubMed]
- Zhang Y, Gu Y, Chen Y, et al. Dingxin Recipe IV attenuates atherosclerosis by regulating lipid metabolism through LXR-α/SREBP1 pathway and modulating the gut microbiota in ApoE mice fed with HFD. Journal of ethnopharmacology. 2021;266:113436. [Crossref] [PubMed]
- Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med 2008;27:2037-49. [Crossref] [PubMed]
- Wang K, Xu D, Yan XL, et al. The impact of primary tumour location in patients undergoing hepatic resection for colorectal liver metastasis. Eur J Surg Oncol 2018;44:771-7. [Crossref] [PubMed]
- Pugh SA, Shinkins B, Fuller A, et al. Site and Stage of Colorectal Cancer Influence the Likelihood and Distribution of Disease Recurrence and Postrecurrence Survival: Data From the FACS Randomized Controlled Trial. Ann Surg 2016;263:1143-7. [Crossref] [PubMed]
- Price TJ, Beeke C, Ullah S, et al. Does the primary site of colorectal cancer impact outcomes for patients with metastatic disease? Cancer 2015;121:830-5. [Crossref] [PubMed]
- Yang CC, Cheng LC, Lin YW, et al. The impact of marital status on survival in patients with surgically treated colon cancer. Medicine (Baltimore) 2019;98:e14856. [Crossref] [PubMed]
- Arnold D, Lueza B, Douillard JY, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol 2017;28:1713-29. [Crossref] [PubMed]
- Gao Z, Guo B, Gao R, et al. Microbiota disbiosis is associated with colorectal cancer. Front Microbiol 2015;6:20. [Crossref] [PubMed]
- Yu T, Guo F, Yu Y, et al. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 2017;170:548-563.e16. [Crossref] [PubMed]
- Missiaglia E, Jacobs B, D'Ario G, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol 2014;25:1995-2001. [Crossref] [PubMed]
- Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med 2015;21:1350-6. [Crossref] [PubMed]
- Allegra CJ, Jessup JM, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol 2009;27:2091-6. [Crossref] [PubMed]
- Roth AD, Tejpar S, Delorenzi M, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol 2010;28:466-74. [Crossref] [PubMed]
- Kalady MF, Dejulius KL, Sanchez JA, et al. BRAF mutations in colorectal cancer are associated with distinct clinical characteristics and worse prognosis. Dis Colon Rectum 2012;55:128-33. [Crossref] [PubMed]
- Loree JM, Pereira AAL, Lam M, et al. Classifying Colorectal Cancer by Tumor Location Rather than Sidedness Highlights a Continuum in Mutation Profiles and Consensus Molecular Subtypes. Clin Cancer Res 2018;24:1062-72. [Crossref] [PubMed]
- Benhattar J, Cerottini JP, Saraga E, et al. p53 mutations as a possible predictor of response to chemotherapy in metastatic colorectal carcinomas. Int J Cancer 1996;69:190-2. [Crossref] [PubMed]
- Wang L, Sun Y, Zhao B, et al. Chemotherapy plus targeted drugs in conversion therapy for potentially resectable colorectal liver metastases: a meta-analysis. Oncotarget 2016;7:55732-40. [Crossref] [PubMed]
- Bagri A, Kouros-Mehr H, Leong KG, et al. Use of anti-VEGF adjuvant therapy in cancer: challenges and rationale. Trends Mol Med 2010;16:122-32. [Crossref] [PubMed]
- van der Wal GE, Gouw AS, Kamps JA, et al. Angiogenesis in synchronous and metachronous colorectal liver metastases: the liver as a permissive soil. Ann Surg 2012;255:86-94. [Crossref] [PubMed]
- Evans C, Morrison I, Heriot AG, et al. The correlation between colorectal cancer rates of proliferation and apoptosis and systemic cytokine levels; plus their influence upon survival. Br J Cancer 2006;94:1412-9. [Crossref] [PubMed]
- Ye SP, Zhu WQ, Liu DN, et al. Robotic- vs laparoscopic-assisted proctectomy for locally advanced rectal cancer based on propensity score matching: Short-term outcomes at a colorectal center in China. World J Gastrointest Oncol 2020;12:424-34. [Crossref] [PubMed]
- Yang B, Huang J, Zhou S, et al. Laparoscopic versus open selective lateral pelvic lymph node dissection following total mesorectal excision for locally advanced low rectal cancer. Int J Colorectal Dis 2020;35:1301-9. [Crossref] [PubMed]
(English Language Editor: A. Muijlwijk)


