
The combination of a prolonged treatment time window and alpha-fetoprotein benefits the tumor response of hepatocellular carcinoma patients as evaluated by the imRECIST: a single-center, retrospective study
Highlight box
Key findings
• The extension of immunotherapy time and the reference to alpha-fetoprotein are beneficial for HCC patients as evaluated by the immune-modified Response Evaluation Criteria in Solid Tumors to Immunotherapy (imRECIST).
What is known and what is new?
• The main advantage of imRECIST over RECIST is that this criterion can help identify possible therapeutic effects in patients with transient PD (less than 12 weeks).
• The use of the imRECIST still has a number of limitations in terms of the analysis of clinical progress and patients’ potential benefits.
What is the implication, and what should change now?
• Alpha-fetoprotein should be included in the efficacy evaluation index to increase the accuracy of imRECIST evaluations.
• Extending the time window of immunotherapy will prolong the overall survival of patients with PD.
Introduction
Liver cancer, of which the most common type is hepatocellular carcinoma (HCC), is the third most common cause of cancer-related death worldwide and represents a deadly threat to human health (1-3). Due to the unique immunogenicity and heterogeneity of HCC, most patients lose the opportunity to undergo radical treatment, as they are in the middle or late stage by the time they show occult clinical manifestations (2). Immune-checkpoint blockades (ICBs) have become one of the most rapidly developed immunotherapy methods in recent years. The safety and therapeutic effectiveness of various ICBs have been shown in a number of experiments (4), and have brought new prospects to the treatment of HCC. With the approval of nivolumab and pembrolizumab for the treatment of HCC (5,6), the treatment of HCC has entered a new era of immunotherapy. Immunotherapy has achieved remarkable results in HCC, and the combination of immune checkpoint inhibitors and molecular targeted drugs was highly anticipated. Superior progression-free survival or overall survival of combination therapy was showed by the Phase III trial of altezolumab and beizumab in advanced HCC (7,8), which undoubtedly confirmed the dual effect of combination therapy on the suppression of tumor growth.
Following the application of these developments in clinical practice, some unconventional and troubling reactions have been observed in the diagnosis and treatment of patients, such as pseudoprogression (9,10). After using ICBs, an initial increase in the tumor burden or the emergence of new lesions, followed by a decrease in the tumor load after further treatment has been observed (11). The current mainstream biological explanation is that due to the reactivation of the immune system, immune cells may flow into the tumor microenvironment, resulting in a temporary increase in inflammation and the tumor load (12,13). Pseudoprogression represents an unconventional but favorable response mode to immunotherapy.
Individual differences in patients’ immune-related response patterns and pseudoprogression have raised additional clinical challenges for doctors and patients, as patients with pseudoprogression may be misclassified with progressive disease (PD) under the conventional Response Evaluation Criteria in Solid Tumors (RECIST) (14) to Immunotherapy (15,16). Due to the errors that arise in evaluating patients treated with immunotherapy using the RECIST or RECIST 1.1 (17), alternate criteria for evaluating the efficacy of solid tumors for immunotherapy patients have been developed, including the immune-related response criteria (irRC) (18), immune-related RECIST (irRECIST) (19), immune RECIST (iRECIST) (20), and immune-modified RECIST (imRECIST) (21). However, improvements can still be made to the response evaluation criteria for solid tumors.
In clinical treatment practice, even if the imRECIST are used, doubts will still arise as to how to balance the risks of clinical progression against the benefits of clinical management (22). More and more attention has being paid to patients who accepted immunotherapy and then confirmed as pseudoprogression. Although the current consensus is that the evaluation cycle can be extended to 4–12 weeks to detect ineffective treatment and rapid disease progression (21). More clinical evidence is still needed to determine whether the assessment cycle needs to be extended for such cases. In addition, there is still a lack of effective biomarkers for the detection and evaluation of the immunotherapy process. Nevertheless, PD-L1 expression levels (23), inflammatory cell related ratios (24), and inflammatory factors (25) have been confirmed to be related to the evaluation of immunotherapy progress in HCC (26). Their corresponding predictive ability still needs to be further verified in future clinical trials. One notable study has shown that immunotherapy delayed the increase of alpha-fetoprotein (AFP), and the AFP response during immunotherapy may indicate the effectiveness of immunotherapy. Surprisingly, patients with AFP response seem to have better survival (27). Other scholars found that AFP is a potential alternative biomarker for the prognosis of HCC patients treated with atelizumab plus bevacizumab (28). It can be seen that the evaluation value of AFP in immunotherapy needs to be explored.
Despite significant advances in the combined immunotargeting therapy (8,29), more clinical research is needed to address the emerging dilemma of immunotherapy, such as developing appropriate tumor response assessment criteria and discovering more noninvasive and practical biomarkers (30). A more appropriate tumor response assessment criteria calls for a fact that imaging evaluation cannot be used in isolation to judge clinical benefit of immunotherapy. Thus, we conducted a retrospective analysis of 32 HCC patients who received immunotherapy plus targeted therapy at the First Affiliated Hospital of Chongqing Medical University from June 2019 to June 2022 to show the necessity of extending the therapeutic time window. The importance of the biomarker, AFP, is emphasized, as its inclusion in the evaluation process will enable more patients to benefit from immunotherapy. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-167/rc).
Methods
Patients and methods
The data of 32 patients with HCC, who had been treated with immunotherapy plus targeted therapy from June 2019 to June 2022 at The First Affiliated Hospital of Chongqing Medical University, were included in this study. ImRECIST was used to evaluate the therapeutic efficacy of the treatment among the patients. To be eligible for inclusion in this study, the patients had to meet the following inclusion criteria: (I) aged 18–75 years; (II) had an Eastern Cooperative Oncology Group (ECOG) score ≤2; (III) were in an unresectable stage; (IV) had a tumor size <10 cm; (V) had a hepatitis B virus (HBV) DNA quantification of <50 IU/mL; (VI) had organs functions that met the following conditions: aspartate aminotransferase (AST) and alanine aminotransferase (ALT) is 5 times less than normal, albumin (ALB) >3 g/dL, total bilirubin <3 mg/dL, creatinine >2 mg/dL, hemoglobin >9 g/dL, neutrophils >1.5×109/L, and platelets >75×109/L. Patients were excluded from the study if they met any of the following exclusion criteria: (I) were undergoing concurrent treatment with any HCC antitumor therapy; (II) had engaged in the long term use of systemic immunosuppressants; (III) had a HBV DNA quantification >50 IU/mL with an active infection (other than a HBV/hepatitis c virus infection) (IV) > grade 2 as defined by the Common Terminology Criteria for Adverse Events; (V) had another serious concomitant disease that could not tolerate treatment; (VI) had a history of other malignant tumors.
All the patients were treated with Bevacizumab (15 mg/kg) and Toripalimab (240 mg) via intravenous infusion every three weeks and no medication is required within three weeks.
The protocol for this human study was approved by the Ethics Committee of The First Affiliated Hospital of Chongqing Medical University, Chongqing, China (No. 2022-K344). All the methods were performed in accordance with the relevant regulations and with the Declaration of Helsinki (as revised in 2013). All the patients provided their written informed consent for the use of their clinical and biological data for the purposes of scientific research upon their enrolment in the study.
Epidemiological data
The following data were collected for each patient: sex, age, ECOG performance status score, Barcelona Clinic Liver Cancer (BCLC) stage, Child-Pugh score, and prior antitumor therapy data (if applicable).
Biological data
Before each immunotherapy cycle, a biological test of blood samples was conducted at the Laboratory of The First Affiliated Hospital of Chongqing Medical University. The following indicators were included in the test: HBV genotype, hemoglobin, platelet count, total bilirubin, ALB, ALT, AST, and AFP.
Evaluation method
Before initial treatment and each immunotherapy cycle, each patient underwent standard abdominal computed tomography (CT) imaging, as well as some biochemical indicators, such as HBV genotype, hemoglobin, platelet count, total bilirubin, ALB, ALT, AST, and AFP. The physical condition to the treatment was periodically measured by biological indicators. The treatment response was defined according to the imRECIST. Overall survival (OS) was defined as the number of days from the first immunotherapy treatment to the date of death or to the date of analysis (i.e., 28 May 2022) if the patient was still alive.
Statistics
Descriptive statistical methods were used to provide epidemiological data and biological index data. A Kaplan-Meier curve was generated to show the relationship between survival time and survival rate. The log-rank test was used to evaluate the differences in the survival outcomes of each treatment group. The results are described as the P value, hazard ratio (HR) and 95% confidence interval (CI). GraphPad Prism 9.3 (GraphPad Software, San Diego, California, America) was used to generate the survival analysis curve for the patients. A P value <0.05, two-sided, was considered statistically significant.
Results
Patient characteristics
The patients’ clinical information at their initial presentation and pre-therapeutic data are summarized in Table 1. A total of 32 patients were included in the study. The patients had a mean age of 51.5 years. Among the 32 patients, 28 (87.50%) were male, and 25 had the HBV. Before the initial immunotherapy, 10 patients had AFP >400 ng/mL. All the patients had ECOG scores of 0. All the patients were in Child-Pugh A. In relation to the BCLC grade, 3 patients had grade A, 14 had grade B, and 15 had grade C. In total, 8 patients did not receive any treatment, 9 patients received comprehensive treatment, 11 patients underwent surgical resection, 2 patients received radio frequency ablation, and 2 patients received transcatheter arterial chemoembolization.
Table 1
| Characteristics | Values |
|---|---|
| Number of patients | 32 |
| Male sex, n (%) | 28 (87.5) |
| Age in years, mean (range) | 51.5 (29 to 67) |
| HBV genotype, n (%) | 25 (78.13) |
| Hemoglobin, mean (range) | 147.5 (85 to 183) |
| Platelet count, mean (range) | 131 (76 to 274) |
| Total bilirubin, mean (range) | 13.9 (6.6 to 29) |
| ALB, mean (range) | 46 (33 to 56) |
| ALT, mean (range) | 29 (17 to 249) |
| AST, mean (range) | 34 (22 to 213) |
| Child-Pugh Score (%) | A (100.0) |
| APF >400 ng/mL, n (%) | 10 (31.3) |
| ECOG PS, score (%) | 0 (100.0) |
| BCLC stage, n (%) | |
| A | 3 (9.4) |
| B | 14 (43.8) |
| C | 15 (46.9) |
| Prior antitumor therapy, n (%) | |
| No therapy | 8 (25.0) |
| Multimodality therapy | 9 (28.1) |
| Resection | 11 (34.4) |
| RFA | 2 (6.3) |
| TACE | 2 (6.3) |
| RECIST, n (%) | |
| PD | 12 (37.5) |
| PR | 8 (25.0) |
| SD | 9 (28.13) |
| CR | 3 (9.38) |
ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AFP, alpha fetoprotein; ECOG PS, Eastern Cooperative Oncology Group performance status; BCLC, Barcelona Clinic Liver Cancer; RFA, radio frequency ablation; TACE, transcatheter arterial chemoembolization; RECIST, Response Evaluation Criteria in Solid Tumors; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Among the patients, 9 (28.13%) achieved stable disease (SD) and 12 (37.5%) showed progressive disease (PD), 3 (9.38%) achieved a complete response (CR), and 8 (25.00%) achieved a partial response (PR). In relation to the 9 patients who achieved SD, 5 patients remained in SD all the time, while 4 were initially in SD at the inception phase of the treatment, but later entered PD showed a constant increase in AFP. In relation to the 12 patients with PD, 8 patients showed sustained PD, and 4 patients were initially in PD but were finally assessed as achieving a PR after treatment. In relation to the 8 patients with a PR, 4 achieved sustained PR, 3 had a period of PR and then achieved SD but were ultimately assessed with PD, and 1 showed a PR after a period, then showed PD, and finally showed a PR.
The target patients included in this study were divided into the following 8 groups: group A (patients with PD); group B (patients with a PR or CR); group C (patients with SD); group D (patients with sustained SD, a sustained PR, or PD that changed to PR); group E (patients with SD that changed to PD, patients with PR changed to SD or PD, patients in sustained PD); group F (patients with sustained SD or PR); group G (patients with PR that changed to PD and then changed to PR, and patients with PD that changed to PR); group H (patients with SD that changed to PD with an increase in AFP and patients with PR that changed to PD with the increase of AFP); and group I (patients with sustained PD). The survival analysis curve of the different tumor treatment responses of patients are shown in Figure 1.
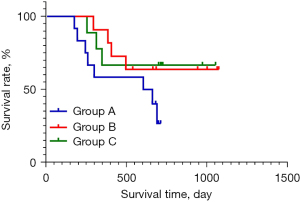
Prolonging the treatment of patients with PD may lead to a PR
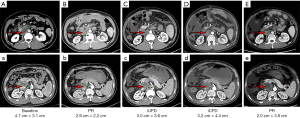
In some patients, PD can be transformed into a PR by providing prolonged treatment, and there is a clinical survival significance. Among the 12 patients with PD, 4 patients benefited from continued treatment after an evaluation of disease progression, and they finally achieved a PR and the occurrence of a disease response at 16 weeks at the latest. An abdominal CT scan of a typical patient is shown in Figure 2. There was an 8-week interval from immune unconfirmed progressive disease to immune confirmed progressive disease (iCPD), and a 16-week interval from iCPD to PR.
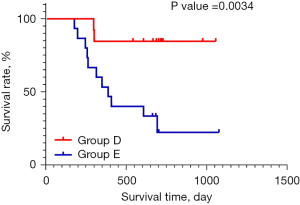
A prolonged therapeutic time window and the administration of continuous medication to patients with PD can prolong their overall survival
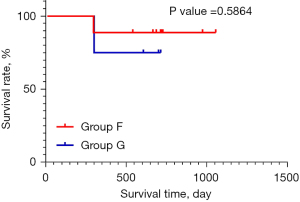
Among the 12 patients with PD, 4 patients benefited from the extension of the treatment therapeutic time window, and with the continuation of the treatment, these patients achieved a PR at 16 weeks at the latest. There was a statistically significant difference in the survival status of patients between group D and group E (P=0.0034, HR =6.842, 95% CI: 2.292 to 20.43; Figure 3); however, no statistical difference was observed in the survival status of patients in group F and group G (P=0.5864; Figure 4).
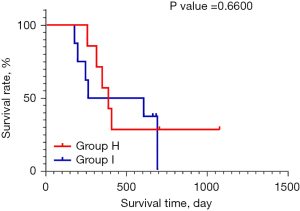
Patients with a PR or SD, but with increased AFP concentrations, ultimately showed PD
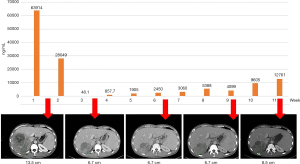
Among the 32 included patients, there were 4 patients with SD and 3 patients with a PR whose AFP concentration continued to increase during treatment, and in whom the disease ultimately progressed. There was no significant difference in the survival of these patients compared to those in a state of continuous PD (P=0.6600; Figure 5). The abdominal CT images and AFP change curves of a typical patient are shown in Figure 6.
Discussion
Compared to the RECIST or RECIST 1.1 revision, the current imRECIST has been shown to have higher accuracy and reliability in evaluating patients after immunotherapy (21,31). However, the current immune evaluation criteria cannot be considered comprehensive, and there are still difficulties in determining disease progression and drug withdrawal in clinical practice. More clinical data need to be gathered to improve and perfect the evaluation criteria. Thus, in this study, we conducted a retrospective analysis of 32 patients with HCC treated by immunotherapy plus targeted therapy at The First Affiliated Hospital of Chongqing Medical University from 2019 to present. The guiding significance of unconventional responses mode was render for clinical actual drug withdrawal and progression judgment. We found that extending the time window of immunotherapy to 16 weeks benefited the patients showed a PD. Using AFP in combination with the imaging tests to determine the treatment effect will increase the accuracy of the results.
HCC has a wide range of intra- and inter-tumoral heterogeneity, progresses rapidly, and has a high degree of malignancy. The liver’s immune response may be more complex than that of other organs (32). More and more single-cell sequencing and multi-omics methods have revealed the heterogeneity and immunosuppression of HCC. Activated NK cells, CD8+ T cells, M1 cells and CD4 memory resting T cells in HCC tissue are the main factors affecting the differences of immunotherapy (33). Immunosuppression-related marker genes and immunosuppressive T cells are also the main causes of poor prognosis. Other studies found that PTPRC and FOXP3 can classify HCC into three subtypes, which are helpful for prognostic prediction and treatment choice (34). These results undoubtedly reveal the heterogeneity and the immune microenvironment of HCC. Therefore, we should attend to the primary and secondary subpopulations in each HCC tumor and take the heterogeneous expression seriously in the process of immunotherapy for HCC, achieving precise treatment (35). According to the survival analysis curve and log-rank test results, there was a significant survival difference between groups D and E, but there was no significant difference between groups F and G. In group G, 5 patients with PD and physical tolerance were willing to continue the treatment after a 12-week observation period (36,37) and thus extend the therapeutic time window. In the process of continuing the treatment, these patients showed unexpected gains, which suggests that there are individual differences in patients’ responses to immunotherapy, the immunization drugs need time to have an effect, and patients’ reaction times may differ.
Due to the difference of immune microenvironment and tumor heterogeneity, the benefits obtained by the patients could not be observed in the traditional 4–12 weeks treatment time window (29,37). However, with the extension of the time of immunotherapy, the body’s immunity started to work patients’ PD status was ultimately reversed to PR. Among the 12 patients with PD, 4 ultimately achieved a PR due to the extension of the treatment time. There was no statistical difference in the survival between these patients and those who maintained a PR or SD all the time. Thus, questions arise as to whether the traditional 4–12-week observation time is sufficient for patients with PD or whether it is necessary to prolong the treatment time window to provide more benefits to patients. The extension of treatment time possibly means that the body has a longer time to develop immune function to fight against tumors.
At present, it is urgent to explore new biomarkers to divine the effectiveness of immunotherapy for HCC. Many studies have shown the importance of biological indicators in determining and predicting tumor progression. For example, the expression of programmed death-ligand 1 is associated with the presence of tumor-infiltrating lymphocytes in the tumor microenvironment (38), which is a favorable prognostic factor for many solid tumors (39). Significant reductions in interleukin-8 levels in the blood during immunotherapy suggest that treatment is effective, and in the absence of such reductions, PD is likely (25,40). In addition, studies have shown that the white-blood-cell/lymphocyte ratio, neutrophil/lymphocyte ratio, and macrophage level are also independent factors that can predict tumor progression after immunotherapy (24,41). Further, some scholars have found that circulating DNA can effectively be used to identify pseudoprogression and hypeprogression (42). Given the small sample size and retrospective nature of this study, further data needs to be gathered to identify the relevant factors.
AFP is an important and recognized serum tumor marker of HCC, which has great significance in the diagnosis, treatment, and efficacy evaluation of HCC (43-46). In our study, we reviewed the final disease status of the patients, and found that even some patients who initially had SD or PR later showed PD as indicated by continuing increases in their AFP levels. In terms of the OS outcomes, no significant difference was observed between group H and group I. An initial status of stable or partial remission for the disease can be likened to an illusion and can delay the treatment of patients. A continuous increase in AFP level, to some extent, may suggest disease progression (47,48), and such a change may not be consistent with the changes observed in tumor imaging examinations.
The imRECIST, which is only based on medical imagining examinations, ignores the disease progress of some patients, and can thus delay changes being made to the method used to treat patients. Notably, for patients with SD, the imRECIST, which is based on medical imaging scans, may not be accurate in determining whether the disease is truly progressing. We found that combining biological indicators with imaging detection to evaluate the disease progress of patients will provide more accurate signals (49). Auxiliary imaging examination and biomarkers are needed to stratify patients and find susceptible groups, so as to achieve accurate selection of treatment time. Thus, we suggest that AFP should be included in the efficacy evaluation index to increase the accuracy of the imRECIST evaluations.
At present, the experimental data that could be used to provide clinical doctors with a reference for making decisions about whether or not to withdraw immunotherapy is limited. Additionally, the problems revealed in the practice of immunotherapy require consideration and discussion from a more comprehensive perspective. Thus, a more comprehensive evaluation of patients is essential. In addition to their radiological progress, a patient’s clinical manifestations, and biological indicator results should be considered in determining whether to continue immunotherapy. Additionally, immunotherapy is heterogeneous, and patients’ responses to immunotherapy may not occur within the expected weeks. Consideration needs to be given as to how the potential benefits of continuing immunotherapy after tumor progression can be balanced against the potential risks of delaying the disease. While extending the treatment time window, Special emphasis should be placed on detecting the trend of changes in AFP and timely imaging follow-up. Given, the limited number of patients in this study, no systematic guidance can be provided. However, our findings warrant attention, and should raise our vigilance, and further steps should be taken to address this issue. Next, we will continue to expand our focus on HCC patients, treated by the immunotherapy and targeted therapy, providing more accurate definitions for the expansion of time windows and the evaluation of efficiency of AFP. The common characteristics of the patients with extended treatment time window will be found through the high throughput screening, multi omics and single cell sequencing, and the biomarker that can evaluate the immunotherapy effect of this similar patients are also developed. We believe that with the wider application of ICBs, more and more convincing research results will be generated, and there will be more clinical benefits for patients.
Conclusions
In summary, our research shows that extending the time window of immunotherapy may prolong the total survival time of more patients. Additionally, the auxiliary role of biochemical indicators in tumor evaluation can not be ignored. The image-based imRECIST, combined AFP, probably ensures an exactitude prediction of HCC patients’condition. All these data indicate that, in a population of patients undergoing immunotherapy, daringly extending the treatment window of immunotherapy and closely monitoring the changes of alpha-fetoprotein will improve the accuracy of prognosis judgment of patients and give patients a profit of survival time.
Acknowledgments
Funding: This study was funded by the National Natural Science Foundation of China (Nos. 81703063, and 81702408), and the Science and Health Joint Research Project of Chongqing Municipality (No. 2020GDRC013).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-167/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-167/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-167/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-167/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The protocol for this human study was approved by the Ethics Committee of The First Affiliated Hospital of Chongqing Medical University, Chongqing, China (No. 2022-K344). All the methods were performed in accordance with the relevant regulations and with the Declaration of Helsinki (as revised in 2013). All the patients provided their written informed consent for the use of their clinical and biological data for the purposes of scientific research upon their enrolment in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Villanueva A. Hepatocellular Carcinoma. N Engl J Med 2019;380:1450-62. [Crossref] [PubMed]
- Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:6. [Crossref] [PubMed]
- Iñarrairaegui M, Melero I, Sangro B. Immunotherapy of Hepatocellular Carcinoma: Facts and Hopes. Clin Cancer Res 2018;24:1518-24. [Crossref] [PubMed]
- El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492-502. [Crossref] [PubMed]
- Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018;19:940-52. [Crossref] [PubMed]
- Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol 2022;76:862-73. [Crossref] [PubMed]
- Galle PR, Finn RS, Qin S, et al. Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): an open-label, randomised, phase 3 trial. Lancet Oncol 2021;22:991-1001. [Crossref] [PubMed]
- Urban H, Steidl E, Hattingen E, et al. Immune Checkpoint Inhibitor-Induced Cerebral Pseudoprogression: Patterns and Categorization. Front Immunol 2021;12:798811. [Crossref] [PubMed]
- Bernard-Tessier A, Baldini C, Castanon E, et al. Patterns of progression in patients treated for immuno-oncology antibodies combination. Cancer Immunol Immunother 2021;70:221-32. [Crossref] [PubMed]
- Chiou VL, Burotto M. Pseudoprogression and Immune-Related Response in Solid Tumors. J Clin Oncol 2015;33:3541-3. [Crossref] [PubMed]
- Di Giacomo AM, Danielli R, Guidoboni M, et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol Immunother 2009;58:1297-306. [Crossref] [PubMed]
- Ellingson BM, Chung C, Pope WB, et al. Pseudoprogression, radionecrosis, inflammation or true tumor progression? challenges associated with glioblastoma response assessment in an evolving therapeutic landscape. J Neurooncol 2017;134:495-504. [Crossref] [PubMed]
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16. [Crossref] [PubMed]
- Billan S, Kaidar-Person O, Gil Z. Treatment after progression in the era of immunotherapy. Lancet Oncol 2020;21:e463-76. [Crossref] [PubMed]
- Le Fèvre C, Lhermitte B, Ahle G, et al. Pseudoprogression versus true progression in glioblastoma patients: A multiapproach literature review: Part 1 - Molecular, morphological and clinical features. Crit Rev Oncol Hematol 2021;157:103188. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009;15:7412-20. [Crossref] [PubMed]
- Nishino M, Giobbie-Hurder A, Gargano M, et al. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res 2013;19:3936-43. [Crossref] [PubMed]
- Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017;18:e143-52. [Crossref] [PubMed]
- Hodi FS, Ballinger M, Lyons B, et al. Immune-Modified Response Evaluation Criteria In Solid Tumors (imRECIST): Refining Guidelines to Assess the Clinical Benefit of Cancer Immunotherapy. J Clin Oncol 2018;36:850-8. [Crossref] [PubMed]
- Cheng L, Xiao H. Sintilimab plus IBI305 for hepatocellular carcinoma. Lancet Oncol 2021;22:e387. [Crossref] [PubMed]
- Carlino MS, Long GV, Schadendorf D, et al. Outcomes by line of therapy and programmed death ligand 1 expression in patients with advanced melanoma treated with pembrolizumab or ipilimumab in KEYNOTE-006: A randomised clinical trial. Eur J Cancer 2018;101:236-43. [Crossref] [PubMed]
- Chasseuil E, Saint-Jean M, Chasseuil H, et al. Blood Predictive Biomarkers for Nivolumab in Advanced Melanoma. Acta Derm Venereol 2018;98:406-10. [Crossref] [PubMed]
- Sanmamed MF, Perez-Gracia JL, Schalper KA, et al. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann Oncol 2017;28:1988-95. [Crossref] [PubMed]
- Gok Yavuz B, Hasanov E, Lee SS, et al. Current Landscape and Future Directions of Biomarkers for Immunotherapy in Hepatocellular Carcinoma. J Hepatocell Carcinoma 2021;8:1195-207. [Crossref] [PubMed]
- Chau I, Park JO, Ryoo BY, et al. Alpha-fetoprotein kinetics in patients with hepatocellular carcinoma receiving ramucirumab or placebo: an analysis of the phase 3 REACH study. Br J Cancer 2018;119:19-26. [Crossref] [PubMed]
- Zhu AX, Dayyani F, Yen CJ, et al. Alpha-Fetoprotein as a Potential Surrogate Biomarker for Atezolizumab + Bevacizumab Treatment of Hepatocellular Carcinoma. Clin Cancer Res 2022;28:3537-45. [Crossref] [PubMed]
- Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol 2021;22:977-90. [Crossref] [PubMed]
- Al-Moundhri M, Cubisino A, Panaro F. Hepatocellular carcinoma with complete response to the immunotherapy: the oncologist's dilemma. Hepatobiliary Surg Nutr 2022;11:119-22. [Crossref] [PubMed]
- Cannella R, Lewis S, da Fonseca L, et al. Immunotherapy-Based Treatments of Hepatocellular Carcinoma: AJR Expert Panel Narrative Review. AJR Am J Roentgenol 2022;219:533-46. [Crossref] [PubMed]
- Kubes P, Jenne C. Immune Responses in the Liver. Annu Rev Immunol 2018;36:247-77. [Crossref] [PubMed]
- Wang T, Dang N, Tang G, et al. Integrating bulk and single-cell RNA sequencing reveals cellular heterogeneity and immune infiltration in hepatocellular carcinoma. Mol Oncol 2022;16:2195-213. [Crossref] [PubMed]
- Zhang Q, Lou Y, Yang J, et al. Integrated multiomic analysis reveals comprehensive tumour heterogeneity and novel immunophenotypic classification in hepatocellular carcinomas. Gut 2019;68:2019-31. [Crossref] [PubMed]
- Ho DW, Tsui YM, Chan LK, et al. Single-cell RNA sequencing shows the immunosuppressive landscape and tumor heterogeneity of HBV-associated hepatocellular carcinoma. Nat Commun 2021;12:3684. [Crossref] [PubMed]
- Hodi FS, Hwu WJ, Kefford R, et al. Evaluation of Immune-Related Response Criteria and RECIST v1.1 in Patients With Advanced Melanoma Treated With Pembrolizumab. J Clin Oncol 2016;34:1510-7. [Crossref] [PubMed]
- Radbruch A, Fladt J, Kickingereder P, et al. Pseudoprogression in patients with glioblastoma: clinical relevance despite low incidence. Neuro Oncol 2015;17:151-9. [Crossref] [PubMed]
- Jiang Y, Lo AWI, Wong A, et al. Prognostic significance of tumor-infiltrating immune cells and PD-L1 expression in esophageal squamous cell carcinoma. Oncotarget 2017;8:30175-89. [Crossref] [PubMed]
- Ruffini E, Asioli S, Filosso PL, et al. Clinical significance of tumor-infiltrating lymphocytes in lung neoplasms. Ann Thorac Surg 2009;87:365-71; discussion 371-2. [Crossref] [PubMed]
- Schalper KA, Carleton M, Zhou M, et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat Med 2020;26:688-92. [Crossref] [PubMed]
- Rosner S, Kwong E, Shoushtari AN, et al. Peripheral blood clinical laboratory variables associated with outcomes following combination nivolumab and ipilimumab immunotherapy in melanoma. Cancer Med 2018;7:690-7. [Crossref] [PubMed]
- Lee JH, Long GV, Menzies AM, et al. Association Between Circulating Tumor DNA and Pseudoprogression in Patients With Metastatic Melanoma Treated With Anti-Programmed Cell Death 1 Antibodies. JAMA Oncol 2018;4:717-21. [Crossref] [PubMed]
- Taketa K. Alpha-fetoprotein: reevaluation in hepatology. Hepatology 1990;12:1420-32. [Crossref] [PubMed]
- Sun X, Mei J, Lin W, et al. Reductions in AFP and PIVKA-II can predict the efficiency of anti-PD-1 immunotherapy in HCC patients. BMC Cancer 2021;21:775. [Crossref] [PubMed]
- Debes JD, Romagnoli PA, Prieto J, et al. Serum Biomarkers for the Prediction of Hepatocellular Carcinoma. Cancers (Basel) 2021;13:1681. [Crossref] [PubMed]
- Watanabe T, Tokumoto Y, Joko K, et al. AFP and eGFR are related to early and late recurrence of HCC following antiviral therapy. BMC Cancer 2021;21:699. [Crossref] [PubMed]
- Wang Q, Li W, Zhang M, et al. α-Fetoprotein fragment synergizes with sorafenib to inhibit hepatoma cell growth and migration and promote the apoptosis. J Cell Mol Med 2022;26:5426-38. [Crossref] [PubMed]
- Kolamunnage-Dona R, Berhane S, Potts H, et al. Sorafenib is associated with a reduced rate of tumour growth and liver function deterioration in HCV-induced hepatocellular carcinoma. J Hepatol 2021;75:879-87. [Crossref] [PubMed]
- Aliyari Ghasabeh M, Shaghaghi M, Pandey A, et al. Integrating baseline MR imaging biomarkers into BCLC and CLIP improves overall survival prediction of patients with hepatocellular carcinoma (HCC). Eur Radiol 2021;31:1630-41. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


