
The emerging role of FAM46C as a biomarker and therapeutic target in gastric adenocarcinoma
Introduction
Gastric adenocarcinoma (GCa) is the third leading cause of cancer-related mortality worldwide and constitutes one of the highest cancer burdens, as measured by disability-adjusted life years lost (1). In North America, five-year survival in patients newly diagnosed with GCa is 29% in Canada (2) and 33% in the United States (3), largely due to distant metastatic disease at diagnosis. Despite favorable initial response to first line systemic regimens, disease progression typically occurs within twelve to eighteen months. Response rates to subsequent lines of treatment remain limited. Of patients who undergo curative-intent resection for their primary GCa, approximately 40% will experience recurrence of disease, despite improvements in surgical and perioperative adjuvant therapy (4). These statistics highlight the need to identify new molecular prognostic and predictive biomarkers, as well as novel therapeutic targets, that are specifically relevant to patients with GCa.
The FAM46/TENT5 family comprises a group of four highly conserved proteins (FAM46A, FAM46B, FAM46C, FAM46D) present in all animal genomes but, until recently, of unknown function and significance (5). Sequence analyses revealed that FAM46 proteins contain the three key motifs found in the nucleotidyltransferase (NTase) fold domain of the NTase superfamily (5), and in vitro experiments demonstrated that all four paralogs are capable of catalyzing polyadenylation (6-8). As such, these genes were renamed as terminal nucleotidyltransferases (TENT5A-D). Unlike canonical poly(A) polymerases (PAPs) such as human poly(A) polymerase gamma (hPAPγ), TENT5A-D do not possess conventional RNA recognition motifs (RRMs) and are thus classified as non-canonical poly(A) polymerase (ncPAP) (9).
The four human FAM46 paralogs are highly similar at the protein sequence level but diverge significantly with respect to DNA sequence and RNA transcription. The paralogs are also subject to differential epigenetic modification at the promoter and coding regions (5). Furthermore, their expression patterns in normal tissues also vary—FAM46A, FAM46B, and FAM46C are expressed in several cell and tissue types, while FAM46D is expressed only in the testis (10). FAM46C mRNA is detected in a wide variety of epithelial tissues, including breast, stomach, respiratory and reproductive tracts, as well as blood cells, including neutrophils and lymphocytes, bone marrow, and mesenchymal tissue (5,10). FAM46C’s ubiquitous expression in normal tissues suggests that it plays a significant biologic role, despite its functions having remained obscure until relatively recently.
The first clue that members of the FAM46 family could be functionally relevant in human cancers emerged with the observation that FAM46C was mutated in ~20% of multiple myeloma patients, a phenomenon that was independently associated with inferior overall survival, suggesting the possibility of a tumor suppressor function for FAM46C (8,11). Subsequently, potentially relevant alterations in FAM46A and FAM46B have been noted in various other cancers. Increased expression of FAM46A is an independent prognostic factor for poor survival in patients with glioblastoma (12) and esophageal adenocarcinoma (13), while decreased expression of FAM46B portends inferior survival in prostate cancer patients (14).
FAM46C is of particular interest due not only to mounting intimations of its capacity to regulate cancer progression in some primary tumor sites (15-17), but also because of an intriguing spectrum of potential molecular mechanisms for its suppression of cancer cell proliferation, invasion and metastasis (18,19). Here, we summarize the current body of literature that implicates FAM46C as a functional tumor suppressor and potential therapeutic target in gastric cancer, reviewing its proposed mechanism(s) of action to suggest avenues for patient selection and response assessment. We present evidence from our own laboratory that indicates the potential for restoration of FAM46C levels to suppress oncogenic polo-like kinase (Plk)4 kinase activity, and thereby restrict gastric cancer progression.
The role of FAM46C in human cancer
The potential tumor suppressive function of FAM46C was first identified in multiple myeloma, where it was eventually attributed to its PAP activity (5,8). Since then, evidence has accumulated to indicate that FAM46C function is relevant to tumorigenesis and tumor progression in a variety of solid tumors, including prostate (16), colorectal (18), and gastric carcinoma (17,20). Moreover, its tumor suppressive function is now thought to be mediated via several non-PAP-related pathways, as will be further discussed below (18,19).
FAM46C in multiple myeloma
Deletions/mutations in FAM46C are common in multiple myeloma. 19% of patients recruited to an early drug trial (n=378) were found to harbor a deletion at 1p12, where the FAM46C gene is located, in their myeloma cells (11). In a whole genome sequencing/whole exome sequencing study of 203 multiple myeloma patients, eleven percent had at least one protein altering FAM46C mutation in their malignant cells, making FAM46C the third most mutated gene in the cohort (21). In another cohort of 114 multiple myeloma patients, deletions and/or mutations in FAM46C were identified in 23% of patients; this was associated with a particularly adverse prognosis (11). While FAM46C deficiency defines patients as at high risk for relapse following treatment, specific implications for targeted therapy remain to be established.
FAM46C in epithelial cancers
Studies in our own laboratory, as well as those published by others (20,22), indicate that significant depletion of FAM46C is very common in GCa tumor tissue compared to normal gastric mucosa; the associated clinicopathologic features and implications of this loss of expression in GCa are detailed below. In colorectal carcinoma (CRC), FAM46C is significantly underexpressed in tumor compared to normal mucosa tissue samples (18). Additionally, FAM46C expression was noted to be lower in CRC of higher clinical stage, particularly so in patients with liver metastasis (18). In prostate cancer, reduced expression of FAM46C in tumor tissue is also associated with inferior overall survival (16). Analysis of The Cancer Genome Atlas (TCGA) dataset shows that FAM46C is significantly underexpressed in hepatocellular carcinoma (HCC) compared to normal liver tissue samples, though the prognostic significance in patients is unclear. Experimentally, forced expression of FAM46C in HCC cells inhibited their proliferation, with induction of a Gap 2 (G2)/mitotic (M) phase arrest, as well as suppression of HCC cell migration and invasion (23). Similarly, in oral squamous cell carcinoma (SCCa), forced expression of FAM46C attenuated proliferation and induced apoptosis in two cell lines (CAL27 and SCC15) that have relatively low basal expression of FAM46C compared to other oral SCCa cell lines. FAM46C knockdown reduced apoptosis and increased proliferation in HSC4 cells, a cell line with relatively high basal FAM46C expression (15). However, the clinical significance of FAM46C in oral SCCa remains unclear.
FAM46C in mesenchymal cancers
Decreased expression of FAM46C has been found in osteosarcoma tumor samples (24). Furthermore, FAM46C expression in osteosarcoma cells was subject to regulation by the oncogenic microRNA, miR-10b.
FAM46C in GCa
To date, three groups have reported analyses of FAM46C expression in GCa specimens and cell lines (17,20,22). In a series of 94 patients who underwent curative intent resection for stage I–III GCa at a single Canadian institution, FAM46C expression was reduced in tumor tissue as compared to paired normal gastric mucosa derived from the same patient in 90% of cases (20) (Figure 1A).
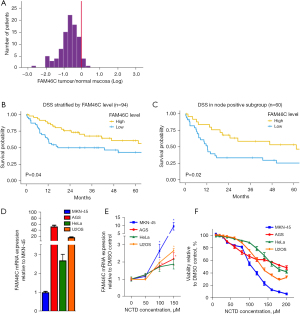
In a series of 129 Japanese patients who had undergone curative-intent resection for GCa, FAM46C expression was noted to be reduced in tumor tissue in 91% of patients (22). Copy number alterations at the FAM46C locus were found in 35% of patients, but did not appear to correlate with FAM46C expression. In this cohort, five-year recurrence-free survival was reduced in patients with low vs. preserved FAM46C expression. Indeed, low FAM46C expression proved to be an independent predictor of inferior recurrence-free survival, with a hazard ratio of 4.6, which was higher than for any other variable, including node status or vessel invasion. Additionally, low FAM46C expression was specifically linked with the development of metachronous hepatic metastases (22). Of note, the long-term survival of patients in this cohort of Japanese patients was exceptionally high (≈75% at five years) compared with what is observed in Western countries. Nevertheless, we found in our Canadian series that FAM46C depletion in the primary tumor was indeed associated with poor survival following curative-intent resection (Figure 1B), with an even stronger prognostic effect in the subset of patients with node positive GCa (Figure 1C) (20).
In a gene set enrichment analysis of 60 GCa tumors and 30 normal tissue samples, Li and co-authors reported a negative correlation between expression of FAM46C and that of genes in the Wnt signaling pathway (17). In vitro experiments using GCa cell lines MKN45 and MKN74 showed that forced FAM46C expression inhibited cell proliferation, inducing a G1 arrest. This occurred in conjunction with a reduction in the proliferative marker Ki-67, as well as decreased β-catenin, MYC, and inhibitor of apoptosis Bcl-2 protein levels (17). As a corollary, knocking down FAM46C in GCa line AGS promoted cell proliferation, increased Ki-67 and Bcl-2 protein levels, and reduced levels of active caspase 3 (17). The authors went on to explore the effects of FAM46C upregulation in vivo, using a human GCa murine xenograft model (17). While there were no phenotypic changes reported, increased FAM46C expression did result in Wnt pathway inhibition. Furthermore, treatment with the Wnt/β-catenin agonist LiCl increased the viability of FAM46C-overexpressing GCa cells, in tandem with an increase in β-catenin and c-MYC expression. These findings suggest that forced expression of FAM46C can be toxic to GCa cells, and that this effect is mediated, at least in part, through inhibition of Wnt/β-catenin signaling (17).
Mechanisms of FAM46C-dependent tumor suppression
In addition to inhibition of the Wnt/β-catenin pathway, there are two broad potential mechanisms through which FAM46C may act as a tumor suppressor in GCa. One relates to the putative NTase activity of FAM46C and the other to its non-transcriptional functions.
NcPAP related functions of FAM46C
Recently, the crystal structures of FAM46C was solved, and, as with other ncPAPs, it possesses a core NTase domain but no RRMs (25). In multiple myeloma cell lines, evidence exists that FAM46C can promote cancer cell death by altering mRNA stability of endoplasmic reticulum (ER)-targeted genes through its NTase activity (8). To date, no such evidence has been found in GCa cell lines, but robust analyses are lacking.
Non-transcriptional functions of FAM46C
Mounting evidence now indicates that FAM46C has functions independent of polyadenylation. For instance, in prostate cancer cells, FAM46C preserves PTEN protein levels by inhibiting ubiquitination without altering PTEN mRNA levels (16). More recently, FAM46C has been shown to directly interact with Plk4, the polo family member known as a master regulator of centriole duplication (18,25). In in vitro experiments, Plk4 directly binds to FAM46C, and the two co-crystallize as a complex, localizing in proximity to the centriole (18,25). Experimental evidence indicates that recruitment of FAM46C to the centriole by Plk4 is crucial for suppressing cell growth in MM1.S multiple myeloma cells (25). In various in vitro migration and invasion assays, FAM46C suppressed these aggressive cancer cell behaviors by inhibiting Plk4 kinase activity (18). In addition, evidence from a murine xenograft model showed that the growth advantage conferred by FAM46C depletion of MDA-MB-435 cells was partially rescued by Plk4 depletion (18), implying a functional interaction whereby FAM46C restrains Plk4’s oncogenic activity in vivo. In several GCa series from Asian centers, artificial intelligence (AI) algorithms have been used to demonstrate that increased Plk4 expression is part of a gene signature associated with particularly poor prognosis (26,27); a combination of Plk4 gain and FAM46C loss could prove to have an even more significant adverse prognostic impact.
“Targeting” FAM46C for cancer treatment
For the most part, new anti-cancer agents that are developed in response to differential gene expression (DGE) analysis will be intended to target proteins and pathways that are apparently upregulated in cancer versus normal tissue. As indicated above, FAM46C is depleted in GCa. Since FAM46C depletion has functional consequences that liberate a variety of oncogenic pathways (16,17,19), restoration of FAM46C function could potentially restrain tumor progression. That said, FAM46C would not be thought of as a conventional “target”. To our knowledge, no work has thus far been done to intentionally develop an agent that would selectively upregulate FAM46C.
Norcantharidin (NCTD) is a synthetic analogue of the traditional Chinese medicine compound cantharidin, itself derived from the Chinese blister beetle, Mylabris phalerata, and historically used to treat a spectrum of non-neoplastic and neoplastic ailments (28). In a screen to determine genes that might mediate the antiproliferative effect of NCTD in hepatoma cells, Zhang et al. described dependence of the latter on upregulation of FAM46C expression (23). NCTD inhibits proliferation, induces apoptosis, inhibits invasion, and exerts anti-angiogenic effects in a variety of cancer cells (28). Indeed, NCTD has recently garnered interest for its anticancer properties as delineated in preclinical and clinical studies [reviewed in (28)]. For GCa specifically, NCTD has been studied in one small clinical trial where postoperative patients were randomized to either conventional cytotoxic chemotherapy alone or chemotherapy plus NCTD; patients who received the combination therapy had a lower rate of cancer recurrence and a higher three-year overall survival rate (29). Currently, NCTD is approved for clinical use only in China, where it is used as an adjunct to chemotherapy or radiotherapy for liver, esophageal, colorectal, and gastric cancers (28). There is currently one open clinical trial that is investigating the safety of NCTD-loaded microspheres injected into patients with solid tumors (30).
A variety of molecular mechanisms have been proposed for the anti-cancer activity of NCTD [reviewed in (28)]. Briefly, NCTD induces apoptosis via the MAPK-ERK pathway in CRC and HCC cells; via the Wnt/β-catenin pathway in medulloblastoma and leukemia; and via the PI3K/Akt pathway in breast cancer and lymphoma. NCTD also suppresses Epithelial-to-mesenchymal transition (EMT) by inhibiting the αvβ6-ERK-ETS1 pathway in colorectal cancer, as well as the Yes-associated protein (YAP) in non-small cell lung cancer (NSCLC), and the Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3)/TWIST pathway in HCC. The potential intersection of these multiple signalling pathways with FAM46C and its downstream effectors has not yet been fully explored. Of note, FAM46C was essential to NCTD’s modulation of ERK signalling, and FAM46C knock down partially inhibited NCTD’s cytotoxic effect in HCC cancer cells (23). To date, NCTD is the only agent that has been shown to upregulate FAM46C expression in cancer cells, though to our knowledge a systematic screen has not been performed.
Experiments from our laboratory show that NCTD increases FAM46C expression in GCa cell lines. Interestingly, baseline levels of FAM46C mRNA expression are higher in AGS GCa cells than in HeLa cervical cancer or U2OS osteosarcoma cells, and strikingly higher (50×) in AGS than in the very aggressive MKN-45 GCa cell line (Figure 1D). NCTD treatment results in a dose dependent increase in FAM46C mRNA levels, as determined by reverse transcription polymerase chain reaction (RT-PCR), both in AGS and MKN-45 cells (Figure 1E). The proportional increase over baseline was greater in MKN-45 cells, though the absolute level of FAM46C expression was still ten-fold greater in AGS than MKN-45 cells at the highest concentration of NCTD tested. NCTD treatment decreased cell viability in both GCa cell lines in a dose dependent manner, and the degree of cytotoxicity correlated with the extent of FAM46C overexpression induced in each cell line vs. its own baseline (Figure 1F).
Based on the above, we hypothesize that FAM46C functions as a tumor suppressor in GCa and that NCTD kills GCa cells by upregulating FAM46C expression. To test this, we knocked down FAM46C expression in AGS cells to 45–55% of control (at the mRNA and protein levels) using shRNA, without any apparent effect on cell viability (Figure 2A,2B). NCTD treatment induced FAM46C expression in control shGFP AGS cells, but clearly failed to increase FAM46C expression in shFAM46C AGS cells (Figure 2C). Cell killing by NCTD was blunted in shFAM46C AGS cells (Figure 2D). This decreased susceptibility to NCTD in FAM46C-depleted AGS cells suggests that the cytotoxic effect of NCTD is mediated at least in part through FAM46C.

Insights into how FAM46C functions as a tumor suppressor in GCa can indicate avenues for other therapeutic strategies. Since FAM46C is an endogenous Plk4 inhibitor, Plk4 could represent a promising target in GCa. Centrinone-B, which is a highly selective inhibitor of Plk4 (31), did not appear toxic to AGS GCa cells at baseline or following FAM46C depletion (Figure 3A,3B). While viability and proliferation may be unaffected, centrinone-B does suppress migration of AGS cells in vitro (Figure 3C). Furthermore, as recently demonstrated, centrinone-B abrogates peritoneal implantation of GCa cells in an ex vivo peritoneal explant model (32), consistent with mediation through Plk4 kinase activity. Of note, treatment with centrinone-B did not impact the reduction in FAM46C mRNA expression induced by siFAM46C (Figure 3D). Furthermore, expression of Plk4 at the mRNA level was not altered by depletion of FAM46C with or without centrinone-B treatment of AGS cells. These findings are consistent with the documented interaction of FAM46C and Plk4 at the protein-protein level, with FAM46C blocking Plk4 kinase activity (18). The role of Plk4 activity in GCa progression requires further investigation, particularly with respect to its interaction with FAM46C. Patients whose tumors harbor unrestrained Plk4 kinase activity due to profound depletion of FAM46C may be most optimally selected for FAM46C repletion.

Conclusions and future perspectives
Of the four FAM46 paralogs that comprise the human FAM46/TENT5 family of putative nucleotidyl transferases, FAM46C has received the most attention as a clinically relevant biomarker. Recent clinical and pre-clinical data support the role of FAM46C as a tumor suppressor in GCa. FAM46C depletion in tumor tissue relative to normal gastric mucosa is common in advanced GCa, and correlates with aggressive GCa cell behavior, as well as an adverse prognosis in patients. Restoration of FAM46C can reverse aggressive GCa phenotypes such as proliferative advantage, migration, and invasion; this reversal is mediated at least in part through suppression of the kinase activity of oncogenic Plk4. The prognostic significance of FAM46C loss should ideally be analyzed in concert with other emerging molecular features; large data sets generated through multicenter collaboration are suitable for AI-guided analysis. The therapeutic potential of norcantharidin, which upregulates the expression of FAM46C in GCa, is under evaluation in clinical trials. Further work is required to uncover and strategically augment the molecular pathways that mediate tumor suppression by FAM46C.
Acknowledgments
Results shown in Figures 1-3 have been published in part in Shelly Luu’s doctoral thesis, year 2021, University of Toronto, Canada. Permission has been obtained from the copyright holder for the reproduction of these materials.
Funding: The original experimental research described herein was supported by
Footnote
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-105/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-105/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Our original experiments described herein were approved by the Research Ethics Board of Sinai Health System, Toronto, Canada (MSH REB No. 16-0084-E) and were conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from all individual participants whose data were entered into the prospectively maintained database. Individual consent for the retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thrift AP, Wenker TN, El-Serag HB. Global burden of gastric cancer: epidemiological trends, risk factors, screening and prevention. Nat Rev Clin Oncol 2023;20:338-49. [Crossref] [PubMed]
- Canadian Cancer Statistics Advisory Committee in collaboration with the Canadian Cancer Society, Statistics Canada and the Public Health Agency of Canada Canadian Cancer Statistics 2023 Toronto, ON: Canadian Cancer Society; 2023 Available online: https://cdn.cancer.ca/-/media/files/research/cancer-statistics/2023-statistics/2023_PDF_EN.pdf
- SEER Program. SEER Cancer Stat Facts: Stomach Cancer Bethesda, MD: National Cancer Institute; Available online: https://seer.cancer.gov/statfacts/html/stomach.html
- Spolverato G, Ejaz A, Kim Y, et al. Rates and patterns of recurrence after curative intent resection for gastric cancer: a United States multi-institutional analysis. J Am Coll Surg 2014;219:664-75. [Crossref] [PubMed]
- Kuchta K, Muszewska A, Knizewski L, et al. FAM46 proteins are novel eukaryotic non-canonical poly(A) polymerases. Nucleic Acids Res 2016;44:3534-48. [Crossref] [PubMed]
- Lin HH, Lo YL, Wang WC, et al. Overexpression of FAM46A, a Non-canonical Poly(A) Polymerase, Promotes Hemin-Induced Hemoglobinization in K562 Cells. Front Cell Dev Biol 2020;8:414. [Crossref] [PubMed]
- Hu JL, Liang H, Zhang H, et al. FAM46B is a prokaryotic-like cytoplasmic poly(A) polymerase essential in human embryonic stem cells. Nucleic Acids Res 2020;48:2733-48. [Crossref] [PubMed]
- Mroczek S, Chlebowska J, Kuliński TM, et al. The non-canonical poly(A) polymerase FAM46C acts as an onco-suppressor in multiple myeloma. Nat Commun 2017;8:619. [Crossref] [PubMed]
- Martin G, Keller W. RNA-specific ribonucleotidyl transferases. RNA 2007;13:1834-49. [Crossref] [PubMed]
- Uhlén M, Fagerberg L, Hallström BM, et al. Proteomics. Tissue-based map of the human proteome. Science 2015;347:1260419. [Crossref] [PubMed]
- Boyd KD, Ross FM, Walker BA, et al. Mapping of chromosome 1p deletions in myeloma identifies FAM46C at 1p12 and CDKN2C at 1p32.3 as being genes in regions associated with adverse survival. Clin Cancer Res 2011;17:7776-84. [Crossref] [PubMed]
- Wang Y, Cai R, Wang P, et al. FAM46A expression is elevated in glioblastoma and predicts poor prognosis of patients. Clin Neurol Neurosurg 2021;201:106421. [Crossref] [PubMed]
- Dong Z, Wang J, Zhan T, et al. Identification of prognostic risk factors for esophageal adenocarcinoma using bioinformatics analysis. Onco Targets Ther 2018;11:4327-37. [Crossref] [PubMed]
- Liang T, Ye X, Yan D, et al. FAM46B Promotes Apoptosis and Inhibits Glycolysis of Prostate Cancer Through Inhibition of the MYC-LDHA Axis. Onco Targets Ther 2020;13:8771-82. [Crossref] [PubMed]
- Zhuang X, Lu M. The potential functions of FAM46C in oral squamous cell carcinoma. Onco Targets Ther 2018;11:8915-23. [Crossref] [PubMed]
- Ma L, He H, Jiang K, et al. FAM46C inhibits cell proliferation and cell cycle progression and promotes apoptosis through PTEN/AKT signaling pathway and is associated with chemosensitivity in prostate cancer. Aging (Albany NY) 2020;12:6352-69. [Crossref] [PubMed]
- Shi J, Zhu Q, Wu J, et al. FAM46C suppresses gastric cancer by inhibition of Wnt/beta-catenin. Front Biosci (Landmark Ed) 2020;25:549-63. [Crossref] [PubMed]
- Kazazian K, Haffani Y, Ng D, et al. FAM46C/TENT5C functions as a tumor suppressor through inhibition of Plk4 activity. Commun Biol 2020;3:448. [Crossref] [PubMed]
- Manfrini N, Mancino M, Miluzio A, et al. FAM46C and FNDC3A Are Multiple Myeloma Tumor Suppressors That Act in Concert to Impair Clearing of Protein Aggregates and Autophagy. Cancer Res 2020;80:4693-706. [Crossref] [PubMed]
- Luu S. FAM46C/TENT5C Is a Tumour Suppressor in Gastric Adenocarcinoma [Ph.D.]. Canada -- Ontario, CA: University of Toronto (Canada); 2021.
- Lohr JG, Stojanov P, Carter SL, et al. Widespread genetic heterogeneity in multiple myeloma: implications for targeted therapy. Cancer Cell 2014;25:91-101. [Crossref] [PubMed]
- Tanaka H, Kanda M, Shimizu D, et al. FAM46C Serves as a Predictor of Hepatic Recurrence in Patients with Resectable Gastric Cancer. Ann Surg Oncol 2017;24:3438-45. [Crossref] [PubMed]
- Zhang QY, Yue XQ, Jiang YP, et al. FAM46C is critical for the anti-proliferation and pro-apoptotic effects of norcantharidin in hepatocellular carcinoma cells. Sci Rep 2017;7:396. [Crossref] [PubMed]
- Gao XZ, Xi XF, Zhang SP. Down-regulation of miR-10b represses cell vitality in osteosarcoma and is inversely associated with prognosis via interacting with FAM46C: Running title: MiR-10b/FAM46C axis modulates OS progression. Tissue Cell 2020;63:101331. [Crossref] [PubMed]
- Chen H, Lu D, Shang G, et al. Structural and Functional Analyses of the FAM46C/Plk4 Complex. Structure 2020;28:910-921.e4. [Crossref] [PubMed]
- Guo SH, Ma L, Chen J. Identification of Prognostic Markers and Potential Therapeutic Targets in Gastric Adenocarcinoma by Machine Learning Based on mRNAsi Index. J Oncol 2022;2022:8926127. [Crossref] [PubMed]
- Hu J, Yu W, Dai Y, et al. A Deep Neural Network for Gastric Cancer Prognosis Prediction Based on Biological Information Pathways. J Oncol 2022;2022:2965166. [Crossref] [PubMed]
- Pan MS, Cao J, Fan YZ. Insight into norcantharidin, a small-molecule synthetic compound with potential multi-target anticancer activities. Chin Med 2020;15:55. [Crossref] [PubMed]
- Zhang L, Xiang H. Clinical efficacy of Norcantharidin combined with conventional chemotherapy treating postoperative gastric cancer. Med Recapitulate. 2013;19:2087-8.
- Liu Y. Phase I Clinical Study for Evaluation of Pharmacokinetic, Safety, Tolerance of Norcantharidin Lipid Microsphere for Injection in Patients With Solid Tumor: ClinicalTrials.gov; [NCT04673396]. Available online: https://ClinicalTrials.gov/show/NCT04673396
- Wong YL, Anzola JV, Davis RL, et al. Cell biology. Reversible centriole depletion with an inhibitor of Polo-like kinase 4. Science 2015;348:1155-60. [Crossref] [PubMed]
- Ng D, Ali A, Lee K, et al. Investigating the mechanisms of peritoneal metastasis in gastric adenocarcinoma using a novel ex vivo peritoneal explant model. Sci Rep 2022;12:11499. [Crossref] [PubMed]


