
Laparoscopic distal pancreatectomy for pancreatic cancer: an overview of evaluation and treatment strategies
Highlight box
Key findings
• The clockwise technique is useful for laparoscopic distal pancreatectomy as it offers wide exposure and a standardized approach to peripancreatic dissection.
• Gradual, step-wise compression of pancreatic parenchyma is crucial.
• Patient positioning is important, as gravity aids greatly in exposure.
What is known and what is new?
• There are benefits of laparoscopic distal pancreatectomy over the open approach.
• The operation is still not universally performed laparoscopically.
• Peripancreatic dissection is facilitated by this technique including dissection of celiac trunk and branches with thorough lymphadenectomy possible through this technique.
• Wide exposure achieved and gravity-aided retraction facilitates dissection as visualization is markedly improved.
What is the implication, and what should change now?
• We recommend a standardized, step-wise approach to laparoscopic distal pancreatectomy. It allows for better anatomic exposure and easier dissection throughout the procedure.
Introduction
Minimally invasive surgery (MIS) has become the standard approach for many abdominal operations, with common benefits including shorter length-of-stay, decreased post-operative pain, and lower incidence of perioperative complications (1,2). Distal pancreatectomy (DP) can be a curative procedure for pathologies of the mid and distal pancreas, with laparoscopic distal pancreatectomies (LDP) providing similar benefits as other MIS procedures (3,4).
Here, we present a brief history of LDP along with anatomical and technical details of the procedure. Although the term “subtotal pancreatectomy” is sometimes used to refer to left-sided resections that extend medially to the pancreatic neck, in this paper the term DP will encompass all resections of the left-sided pancreas. This includes resections that involve the neck, body, and/or tail of the pancreas.
As MIS DP becomes standard of care (4), the authors offer a salient overview of historical context and important technical details.
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional board of Miami Cancer Institute and individual consent for this retrospective analysis was waived.
Historical context
The first DP (and first known anatomic pancreatic resection) was performed in 1882 by Friedrich Trendelenburg for spindle cell carcinoma (5). By the mid-20th century case reports of successful DP were more prevalent, and the procedure eventually became an important part of the pancreatic surgeon’s armamentarium. In 1994 Alfred Cuschieri was the first to publish reports of LDP (6), with larger series appearing in literature by the early 2000’s (7,8).
Multiple series and subsequent meta-analyses began showing benefits to LDP over open DP (ODP) (9-13). The most common advantages of LDP found include decreased need for blood transfusion, faster functional recovery, and decreased hospital length of stay. Many patients in these studies carried a malignant diagnosis. These studies find no significant differences in morbidity, mortality, or oncologic outcomes in patients with malignancies.
The landmark LEOPARD trial was a Dutch randomized controlled trial that helped solidify the benefits of LDP (14). In this double-blinded, multicenter trial, patients who underwent MIS DP as compared to ODP had shorter functional recovery, less intraoperative blood loss, less delayed gastric emptying, and improved postoperative quality of life. Forty-two of the 47 patients in the MIS cohort underwent LDP, and the remaining 5 underwent robotic DP. There was no difference in oncologic outcomes between the two cohorts in those patients with pancreatic ductal adenocarcinoma. A second randomized controlled trial in Sweden compared LDP to ODP, with similar results (15).
An MIS approach has been advocated as the standard of care for DP. The Miami International Evidence-based Guidelines on Minimally Invasive Pancreatic Resection set forth recommendations related to MIS DP in 2020 after a multi-society, multinational consensus meeting (4). It provides strong recommendations to perform MIS DP for benign and low-grade malignant lesions over ODP. This includes no contraindications for an MIS approach based on patient’s age, obesity, or surgical history. The guidelines found that MIS DP for pancreatic ductal adenocarcinoma is “feasible, safe, and oncologically efficient”. Robotic-assisted approaches are less well-studied, but outcomes appear to be equivalent. These recommendations were upheld during a re-evaluation of updated literature and publishing of subsequent international guidelines, termed the Brescia Guidelines, in 2024 (16).
Indications
LDP is performed for one of four general indications: malignant/suspicious lesions, benign lesions with malignant potential, hormonally active lesions, and as management of complications from pancreatitis or pancreatic trauma. Examples of premalignant lesions include certain cystic lesions, such as intraductal papillary mucinous neoplasms or mucinous cystic neoplasms. Complications from pancreatitis or trauma that may require surgical intervention include persistent pancreatic fistulas, pancreatic ductal disruption, chronic pain with known focus of chronic pancreatitis. Management of malignancies and many benign or premalignant lesions should routinely be discussed at multidisciplinary hepatopancreatobiliary tumor board meetings.
Anatomic pearls
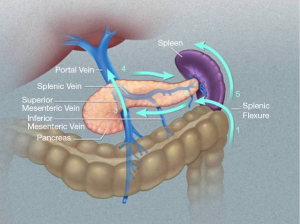
The superior mesenteric artery (SMA) and vein (SMV) mark the anatomic boundary between the head and uncinate process to the right and the neck, body, and tail to the left. The neck of the pancreas lies directly over the SMA/SMV, with the body and tail to its left.
The duodenum and ligament of Treitz are identified along the inferior aspect of the neck/body of the pancreas.
The splenic artery generally originates near the neck of the pancreas and runs along its upper border. Branches of the splenic artery supply the distal pancreas. The splenic vein courses inferior and at times posterior to the splenic artery. Most commonly, the splenic vein joins with the SMV to form the hepatic portal vein, with the inferior mesenteric vein (IMV) inserting into the splenic vein. However, there is much anatomic variation. Review of these and other anatomic variations is critical in operative planning.
The pancreas is entirely retroperitoneal. A summary of the pertinent anatomic relationships is as follows: splenic artery at superior edge of pancreas, splenic vein posterior to pancreas, and colonic mesentery inferior to pancreas and often abutting it. Deep to the pancreas lies Gerota’s fascia, adrenal gland, and kidney laterally, with renal vein more medial.
More specifically, the pancreas lies in the anterior pararenal space, with the parietal peritoneum anterior to the pancreas and Gerota’s fascia posterior. The transverse colon, splenic flexure, and descending colon run inferior/lateral to the pancreas. An important plane is identified during surgery between the colonic mesentery and fat from the adjacent pararenal space. The tail of the pancreas continues into the splenorenal ligament as it nears the splenic hilum. This ligament essentially encompasses a lateral area of thinned pararenal space and is made of peritoneum both anteriorly and posteriorly. The splenorenal ligament is continuous with the gastrosplenic ligament superiorly, which contains the short gastric vessels.
The degree of fatty tissue within the pararenal space varies amongst patients, and careful dissection with frequent observation of nearby structures is important to ensure the correct planes are followed. Failure to do so could lead to inadvertent injury to the adrenal gland, left kidney, or renal hilum.
Preoperative considerations
Proper workup and planning of patients before pancreatic resection are crucial. A full history and physical is important to assess severity of symptoms and any clinical manifestations attributable to the primary pathology. This possibly includes workup for pancreatic insufficiency (diabetes, malabsorption), systemic disease, hormonal hyperactivity, depleted nutritional status, or other underlying problems such as biliary or foregut pathology that may have overlapping signs and symptoms. Routine laboratory testing should include a complete blood count, a comprehensive metabolic panel including liver enzymes, coagulation panel, and in the case of malignancy serum carbohydrate antigen 19-9 (CA 19-9) and carcinoembryonic antigen (CEA) tumor marker levels.
Tissue diagnosis prior to resections of suspicious lesions is common but not always necessary, and decisions must be individualized for each specific situation. For example, a pancreatic tail lesion suspicious for an early-stage malignancy may be amenable to up-front resection without biopsy. However, that same lesion at a more advanced stage might qualify for neoadjuvant chemotherapy, in which case tissue sampling is mandatory for pathologic diagnosis and molecular analysis. Biopsies are preferentially obtained through endoscopic ultrasound-guided biopsy. Image-guided percutaneous biopsy and other methods are at times alternatively employed.
Also imperative in presurgical planning is close examination of cross-sectional imaging. Standard of care dictates evaluation with a high-resolution computed tomography (CT) with thin-cut pancreatic protocol contrast phases, or magnetic resonance imaging (MRI) with pancreatic protocol. Endoscopic ultrasound is often an important adjunct, and it is routinely performed during the workup of many pancreatic lesions.
Different pancreatic lesions have characteristic appearances on imaging and the diagnosis is often made based on imaging alone, especially of benign lesions. In suspected malignant lesions, tumor relationship to surrounding anatomic structures, especially vasculature, must also be carefully examined. Major vessel involvement may require vascular resection/reconstruction or may deem a patient ineligible for resection. It is useful to review the imaging and conceptualize which paths of dissection and transection will be taken. This allows for adequate mental preparation for what one may encounter intraoperatively.
It is crucial for patients to be evaluated at centers with a high volume of pancreatic resections. Clinical decisions should be made in a multidisciplinary fashion at formal tumor boards. Multiple investigations support better outcomes when patients are evaluated and treated in this context (17-21).
Further preoperative planning involves nutritional optimization and patient physical prehabilitation, adequate control of comorbidities including diabetes and pancreatic insufficiency, and appropriate cardiopulmonary workup. Bowel preparation should be considered if segmental colonic resection is a possibility. Detailed planning with the anesthesiology team is important to assess the need for central venous and arterial line placement, anticipated blood loss, and adjuncts to pain control with an emphasis on avoiding narcotics. Perioperative antibiotics, post-splenectomy vaccines, and thromboprophylaxis are administered as per the standard of care.
Results
Operative approach
Technique
The authors approach LDP using the “clockwise technique”. This approach to LDP was developed by Horacio J Asbun and has been used extensively with favorable long-term results (9,22). The technique proceeds from lateral to medial and from caudad to cephalad, and then medial to lateral (Figure 1). It is described as follows.
Key steps
- Patient positioning and trocar placement.
- Mobilization of the descending colon and splenic flexure, entry into the lesser sac.
- Takedown of the gastrosplenic and gastrocolic ligaments for full exposure of the lesser sac and pancreas.
- Lateral to medial dissection along the inferior aspect of the pancreas.
- Retropancreatic dissection, stapled pancreatic and splenic vascular transection using stepwise compression.
- Medial to lateral dissection along the superior aspect of the pancreas.
- Splenic mobilization, specimen retrieval.
Positioning
Positioning is an important consideration, as gravity is used to assist with exposure during reflection of anatomic planes. The patient is well secured to the operating table with arms out and padding of pressure points. They are either supine or in a modified right semi-lateral position (15°–30°), with the degree of rotation dependent on the location of the lesion. Patients with more central lesions are generally more supine. The table is adjusted as needed intraoperatively for more or less lateral tilting. Patients are usually also in a substantial reverse Trendelenburg position.
Trocar placement
The surgeon stands to the patient’s right side. The standard approach employs four surgical trocars: two 12 mm trocars and two 5 mm trocars (Figure 2). A 5 mm optical trocar with insufflation is used to enter the abdomen in the supraumbilical region, about 16 cm from the xyphoid process. This trocar is later upsized for a 12 mm trocar. Alternatively, Hassan open entry technique can be used to place this initial supraumbilical trocar. After insufflation, a 5 mm trocar is placed laterally about 2 cm below the subcostal margin on the patient’s left side. Another 12 mm trocar is placed between the initial two, approximately 8 cm to the left of the first 12 mm trocar. An epigastric 5 mm trocar is placed to the right of the midline, roughly in-line with the inferior edge of the liver. This fourth trocar is adjusted to the patient’s right for more central lesions, or to the patient’s left in case of a more obese abdomen, which facilitates access to the superior aspect of the spleen. An additional 5 mm trocar may be inserted along the right subcostal margin to facilitate retraction of an enlarged stomach (using a fixed liver retractor), although this is rarely necessary.

In obese patients, it is helpful to position the trocars more cephalad and along the left subcostal region. They are placed approximately 5 cm below the costal margin in an arc configuration, centered around the location of the lesion to be resected. This allows the surgeon to approach the lesser sac in more of an anterior-posterior direction, with the right and left hands triangulating around the camera/lesion.
Dissection: lateral to medial
The key to exposure is early, wide dissection. An energy device of surgeon’s preference is used to mobilize the descending colon and splenic flexure along the white line of Toldt. As the splenic flexure is taken down, the avascular plane between the colonic mesentery and surrounding retroperitoneal fat is separated with blunt dissection in a cephalad direction and to the patient’s right. The lesser sac is entered from lateral to medial. If there is fusion of tissue planes, the gastrocolic ligament can be incised somewhat cephalad and medial to the splenic flexure, with care to avoid injuring the splenic hilum. Strong but careful retraction with the nondominant hand coupled with gravity from proper positioning aid significantly in gaining adequate exposure. Dissection is precise, in order to avoid dissecting away from the avascular plane and into retroperitoneal fat or Gerota’s fascia posteriorly.
Dissection with the energy device is continued through the gastrosplenic ligament to take down the short gastric vessels of the stomach. The spleen is not mobilized. The gastrocolic ligament is further incised from lateral to medial, remaining outside the gastroepiploic. This assists in mobilizing the stomach medially, and it is rolled anteromedially under the left liver and out of the operative field. The pancreas becomes readily apparent in the retroperitoneum of the lesser sac.
The authors have found that using intraoperative fluorescence imaging with indocyanine green (ICG) as a continuous infusion can be of great help when delineating the edges of the pancreas (Figure 3) (24). The infusion produces fluorescence of the pancreatic parenchyma without significant background fluorescence from the liver or adjacent organs. However, there are patient factors such as pancreatic fibrosis or fatty infiltration that can affect visualization with ICG.

The peritoneum overlying the inferior edge of the pancreas is incised laterally and dissection continues along the inferior border of the pancreas in a lateral to medial direction, close to the pancreatic parenchyma, allowing for inferior gravity retraction of the transverse mesocolon. Based on the assessment of preoperative imaging, the surgeon should be aware of the location of the IMV, ligament of Treitz, and duodenum as they continue along the inferior border of the pancreas. The IMV may need to be isolated and divided depending on its course.
Pancreatic transection
At this point, the lesion to be resected should be easily identifiable, with employment of intra-operative ultrasound as needed. The desired site of pancreatic transection is established with clear margins medially.
A retropancreatic tunnel is created with careful blunt dissection at the site of future transection. A Penrose drain or vessel loop is passed through the tunnel to aid in anterior retraction of the pancreas. A 60 mm laparoscopic linear stapler with absorbable staple line reinforcement is passed into the retropancreatic tunnel. Staplers with 2.0 mm closed staple height are generally adequate for parenchymal transection including en-bloc transection of the splenic vessels, although the surgeon should adjust the size of the staple load if the pancreas is felt to be abnormally thick or thin.
If the lesion is in the neck of the pancreas, the splenic artery and vein will likely need to be dissected separately given the proximity to the celiac axis/portal vein and transected with separate vascular-load cartridges (0.75 mm). In this case, the parenchyma is divided with a 1.5 or 1.0 mm cartridge, depending on its thickness.
Stepwise compression of the stapler is important to avoid parenchymal fracture and increased risk of pancreatic fistula. The stapler is slowly closed until resistance is felt, at which time compression is paused for approximately 20 seconds before proceeding. It is thus closed incrementally in a stepwise over several minutes. If necessary, transection can be carried out through more than one staple fire, starting with the inferior portion of the pancreas and then the upper aspect (including splenic vessels).
Dissection: medial to lateral
Dissection then continues along the superior edge of the pancreas, including residual posterior attachments. The pancreas is retracted anteriorly using an open bowel grasper during transection to aid in visualization and continued exposure of the appropriate plane. This superior dissection begins medially at the point of pancreatic transection and continues laterally towards the spleen, which has not yet been mobilized. This again proceeds adjacent to pancreatic parenchyma, in the plane between the posterior pancreas and Gerota’s fascia.
Once beyond the tail of the pancreas, the attachments of the spleen are finally taken down. At this point, the distal pancreas is freely mobile and widely exposed, providing easy access to the splenic attachments. Division of splenic attachments generally begins inferiorly and is carried laterally, and then superiorly.
Specimen retrieval
The entire specimen is placed in large retrieval bag, with the pancreas oriented near the bag opening. It is removed from the midline 12 mm trocar site or the left lateral incision, usually with need for extension of the incision. For abnormally large specimens, a Pfannenstiel incision can be used. Orienting the pancreatic margin towards the bag opening aids in preserving the margin during removal for intraoperative frozen section and subsequent pathologic examination. It also allows for removal through a smaller incision: once the pancreas is delivered, it can be separated from the spleen at the hilum and the spleen morcellated for easier retrieval.
Final intra-abdominal inspection is performed to assure staple-line hemostasis and clean pancreatic transection. Drains are not routinely used. However, in cases of clearly fractured pancreatic parenchyma with concerns for postoperative pancreatic leak and fistula, a drain may be left in place near the staple line.
Discussion
Alternative approaches
In cases of pancreatic adenocarcinoma or other more invasive lesions, one should consider a wider lymphadenectomy. This usually involves a radical antegrade modular pancreaticosplenectomy (RAMPS) procedure (25). The procedure describes early medial dissection, pancreatic transection, and control of vasculature to allow for skeletonizing the celiac trunk, SMA, and lateral aorta for an N1 lymph node dissection. Dissection then proceeds laterally deep to Gerota’s fascia, and either anterior to the adrenal gland (“anterior RAMPS”) or posterior (“posterior RAMPS”) depending on preoperative imaging and extent of tumor invasion. The reported benefits are high lymph node harvest and high R0 resection rates.
We have found it feasible to perform a laparoscopic “modified RAMPS” in cases of more invasive lesions, which combines the clockwise technique with a deeper, wider lymph node harvest. This allows for radical clearance of lymphatic tissue along the SMA as needed. After exposure, lateral-to-medial mobilization, and parenchymal transection in the clockwise technique, the medial-to-lateral mobilization is undertaken deep to Gerota’s fascia and anterior/posterior to the adrenal gland as indicated. Evaluation of outcomes in a twelve-year review shows equivalent lymph node harvest and R0 resection rates (22).
Surgeons should have a low threshold for performing an open operation in cases of expected increased difficulty. Examples include pancreatic adenocarcinoma with significant vascular involvement or extensive local. Marked inflammation from pancreatitis or traumatic disruption may also warrant an open approach. Contraindications to MIS must also be ruled out in general, which include inability to tolerate pneumoperitoneum, untreated coagulopathy, and known extensive intra-abdominal scarring.
Splenic preservation is also a possibility in benign lesions, although it may increase the complexity of the operation and its benefits are a subject of debate. It requires careful preservation of the splenic artery and vein, with ligation of branches/tributaries, and separation of the pancreas from the splenic vessels. An alternate technique involves transection of the splenic vessels at the pancreatic transection line and separately at the splenic hilum, with preservation of the short gastric vessels and gastroepiploic arcade for perfusion of the spleen (Warshaw technique). The Warshaw technique is touted by its proponents for its relative simplicity when attempting to preserve the spleen.
Although there are no prospective studies directly comparing the clockwise technique to the more traditional medial-to-lateral dissection, a comparison of historical outcomes shows the clockwise technique to be at least as good as the medial-to-lateral technique. In a review of patients who underwent the clockwise technique by two surgeons over a twelve-year period, the rate of clinically significant postoperative pancreatic fistula was 7.1% with 9.9% rate of major morbidity (22). In that study, patients with pancreatic adenocarcinoma had a 93.3% R0 resection rate and median lymph node harvest of 22 [5–48]. This compares favorably with published outcomes of other techniques, such as a traditional RAMPS (overall R0 rate of 81%, median lymph node harvest of 18) and other accepted techniques for MIS DP (R0 rate of 76%, median 15 lymph nodes) (26,27).
Postoperative management
Patients are generally admitted to the hospital postoperatively for two to four days. Nasogastric tubes and Foley catheters are not routinely left in place. Early ambulation is encouraged. A clear liquid diet can be started on post-operative day 1, and advanced as tolerated.
Careful attention must be given to postoperative signs of pancreatic insufficiency, including blood glucose derangements and exocrine pancreatic enzyme deficiency. This is not a common occurrence but can have serious consequences. An in-hospital endocrinology service can be consulted for recommendations on managing postoperative diabetes mellitus.
Postoperative complications include pancreatic leak/fistula and abscesses, which are most often diagnosed on CT and managed through percutaneous drainage. Clinical signs of postoperative leak include abdominal pain (at times referred to the left shoulder), leukocytosis, fever, and ileus. Overwhelming post-splenectomy sepsis is another rare but dangerous infectious complication.
Signs of postoperative bleeding must be swiftly investigated and addressed. Treatment for postoperative hemorrhage is usually accomplished with the assistance of interventional radiologists and endovascular embolization techniques.
Pearls and pitfalls
- Noting the insertion of the IMV into the splenic vein or SMV preoperatively is important. If the IMV inserts into the splenic vein distal to the line of pancreatic transection, it must usually be isolated and ligated separately during dissection.
- When using a manual advancing stapler for pancreatic transection, maintain a steady position on the stapler to avoid moving it during transection. This sawing or rocking motion can result in parenchymal fracture and postoperative leak.
- Avoid straying from the avascular planes. This can result in injury to left renal or adrenal vasculature, the left ureteral, colonic mesentery, or mesenteric/portal venous vessels.
- It is crucial to avoid “tunnel vision” by routinely re-evaluating visible anatomic structures and landmarks. Pancreatic anatomy is difficult and retroperitoneal dissection can be disorienting.
- Management of pseudocysts from pancreatitis is often better managed by drainage procedures. Decision making is multifactorial, but patients that may benefit from pancreatic resection include those with a disrupted pancreatic duct and a small volume of pancreas to be removed, especially in the setting of splenic vein thrombosis.
- A hybrid laparoscopic-open approach can be used for more complex procedures, including total pancreatectomies and Appleby procedure. An MIS approach to distal pancreatic mobilization often allows for better visualization and easier mobilization than would be seen in an open approach to the distal pancreas.
- Without the routine use of surgical drains, it is not uncommon to incidentally find small fluid collections on post-operative imaging. In our experience, these collections most commonly do not have clinical consequence. We only pursue drainage of the fluid in patients with large fluid collections or who are symptomatic. In those cases, drainage can be percutaneous or transgastric.
Conclusions
We present a standardized approach to LDP. Proper understanding of LDP technique is important and helps improve outcomes for the operation. LDP should be a standard option for patients necessitating distal pancreatic resection for cancer.
Acknowledgments
Funding: None.
Footnote
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-784/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-784/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-784/coif). J.A.S. serves as an unpaid editorial board member of Journal of Gastrointestinal Oncology from February 2024 to January 2026. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional board of Miami Cancer Institute and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mohiuddin K, Swanson SJ. Maximizing the benefit of minimally invasive surgery. J Surg Oncol 2013;108:315-9. [Crossref] [PubMed]
- Antoniou SA, Antoniou GA, Antoniou AI, et al. Past, Present, and Future of Minimally Invasive Abdominal Surgery. JSLS 2015;19:e2015.00052.
- Lyu Y, Cheng Y, Wang B, et al. Assessment of laparoscopic versus open distal pancreatectomy: a systematic review and meta-analysis. Minim Invasive Ther Allied Technol 2022;31:350-8. [Crossref] [PubMed]
- Asbun HJ, Moekotte AL, Vissers FL, et al. The Miami International Evidence-based Guidelines on Minimally Invasive Pancreas Resection. Ann Surg 2020;271:1-14. [Crossref] [PubMed]
- Witzel O. Aus der Klinik des Herrn Prof. Trendelenburg. Beiträge zur Chirurgie der Bauchorgane. Deutsche Zeitschrift für Chirurgie 1886;24:326-54. [Crossref]
- Cuschieri A. Laparoscopic surgery of the pancreas. J R Coll Surg Edinb 1994;39:178-84. [PubMed]
- Palanivelu C, Shetty R, Jani K, et al. Laparoscopic distal pancreatectomy: results of a prospective non-randomized study from a tertiary center. Surg Endosc 2007;21:373-7. [Crossref] [PubMed]
- Griffin JF, Poruk KE, Wolfgang CL. Pancreatic cancer surgery: past, present, and future. Chin J Cancer Res 2015;27:332-48. [PubMed]
- Asbun HJ, Van Hilst J, Tsamalaidze L, et al. Technique and audited outcomes of laparoscopic distal pancreatectomy combining the clockwise approach, progressive stepwise compression technique, and staple line reinforcement. Surg Endosc 2020;34:231-9. [Crossref] [PubMed]
- Kim SC, Park KT, Hwang JW, et al. Comparative analysis of clinical outcomes for laparoscopic distal pancreatic resection and open distal pancreatic resection at a single institution. Surg Endosc 2008;22:2261-8. [Crossref] [PubMed]
- Jayaraman S, Gonen M, Brennan MF, et al. Laparoscopic distal pancreatectomy: evolution of a technique at a single institution. J Am Coll Surg 2010;211:503-9. [Crossref] [PubMed]
- Xie K, Zhu YP, Xu XW, et al. Laparoscopic distal pancreatectomy is as safe and feasible as open procedure: a meta-analysis. World J Gastroenterol 2012;18:1959-67. [Crossref] [PubMed]
- Venkat R, Edil BH, Schulick RD, et al. Laparoscopic distal pancreatectomy is associated with significantly less overall morbidity compared to the open technique: a systematic review and meta-analysis. Ann Surg 2012;255:1048-59. [Crossref] [PubMed]
- de Rooij T, van Hilst J, van Santvoort H, et al. Minimally Invasive Versus Open Distal Pancreatectomy (LEOPARD): A Multicenter Patient-blinded Randomized Controlled Trial. Ann Surg 2019;269:2-9. [Crossref] [PubMed]
- Björnsson B, Larsson AL, Hjalmarsson C, et al. Comparison of the duration of hospital stay after laparoscopic or open distal pancreatectomy: randomized controlled trial. Br J Surg 2020;107:1281-8. [Crossref] [PubMed]
- Abu Hilal M, van Ramshorst TME, Boggi U, et al. The Brescia Internationally Validated European Guidelines on Minimally Invasive Pancreatic Surgery (EGUMIPS). Ann Surg 2024;279:45-57. [PubMed]
- Al-Hawary MM, Francis IR, Chari ST, et al. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the society of abdominal radiology and the american pancreatic association. Gastroenterology 2014;146:291-304.e1. [Crossref] [PubMed]
- Tempero MA, Malafa MP, Chiorean EG, et al. Pancreatic Adenocarcinoma, Version 1.2019. J Natl Compr Canc Netw 2019;17:202-10. [Crossref] [PubMed]
- Lieberman MD, Kilburn H, Lindsey M, et al. Relation of perioperative deaths to hospital volume among patients undergoing pancreatic resection for malignancy. Ann Surg 1995;222:638-45. [Crossref] [PubMed]
- van Heek NT, Kuhlmann KF, Scholten RJ, et al. Hospital volume and mortality after pancreatic resection: a systematic review and an evaluation of intervention in the Netherlands. Ann Surg 2005;242:781-8, discussion 788-90. [Crossref] [PubMed]
- Katz MH, Wang H, Fleming JB, et al. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol 2009;16:836-47. [Crossref] [PubMed]
- Dutcher JS, Asbun D, Tice MP, et al. Updated outcomes using clockwise technique for laparoscopic distal pancreatectomy: Optimal treatment of benign and malignant disease of the left pancreas. Laparosc Endosc Robot Surg 2021;4:9-13. [Crossref]
- Asbun HJ, Stauffer JA. Laparoscopic approach to distal and subtotal pancreatectomy: a clockwise technique. Surg Endosc 2011;25:2643-9. [Crossref] [PubMed]
- Asbun D, Kunzler F, Marin R, et al. Pancreatic fluorescence using continuous indocyanine green infusion. J Surg Oncol 2022;126:1215-8. [Crossref] [PubMed]
- Strasberg SM, Linehan DC, Hawkins WG. Radical antegrade modular pancreatosplenectomy procedure for adenocarcinoma of the body and tail of the pancreas: ability to obtain negative tangential margins. J Am Coll Surg 2007;204:244-9. [Crossref] [PubMed]
- Mitchem JB, Hamilton N, Gao F, et al. Long-term results of resection of adenocarcinoma of the body and tail of the pancreas using radical antegrade modular pancreatosplenectomy procedure. J Am Coll Surg 2012;214:46-52. [Crossref] [PubMed]
- Abu Hilal M, Richardson JR, de Rooij T, et al. Laparoscopic radical 'no-touch' left pancreatosplenectomy for pancreatic ductal adenocarcinoma: technique and results. Surg Endosc 2016;30:3830-8. [Crossref] [PubMed]


