
Four clamp-crush techniques in robotic hepatectomy (with video)
Highlight box
Surgical highlights
• Our four types of clamp-crush techniques of robotic hepatectomy are Clamp, Peck, Open, and Sweep. The clamp is the most standard technique, and a hepatic parenchymal transection was performed by crushing the parenchyma and isolating the vessels and the bile ducts. The Peck is the technique in which the forceps are pulled backward without completely closing them. The Open technique is the technique in which the forceps are opened above and below the vessels and bile ducts; it is often used in laparotomies. The sweep is the technique in which partially exposed vessels and bile ducts are rubbed with forceps, allowing the clear exposure of the vessels and bile ducts.
What is conventional and what is novel/modified?
• In a laparoscopic hepatectomy, various energy devices are used for parenchymal transections, especially the clamp-crush method and the Cavitron Ultrasonic Surgical Aspirator (CUSA) system are popular; however, there is no CUSA that can be operated from the robot console.
• Our study is the first report describing the clamp-crush techniques in robotic hepatectomy. We classified conventional clamp-crush methods into four types and verbalized them.
What is the implication, and what should change now?
• The spread of effective clamp-crush techniques in robotic hepatectomy will lead to the spread of robotic hepatectomy.
Introduction
Since the first successful application of robotic hepatectomy in 2003 (1), the number of reported robotic hepatectomy cases has gradually increased.
In a laparoscopic hepatectomy, various energy devices are used for the parenchymal transection of the liver, depending on the institution and the operator. Among various methods, the clamp-crush method and the Cavitron Ultrasonic Surgical Aspirator (CUSA) system are popular, and there are no differences between these two methods in terms of operation time, bleeding volume, or perioperative complications (2-4). However, at present, there is no CUSA that can be operated from the robot console.
We have mainly used the clamp-crush method to perform laparoscopic hepatectomy. The technique generally referred to as the clamp-crush method is not actually a single movement. We believe that the clamp-crush method can be classified into four categories and have tried to verbalize them. In this report, we aim to explain the four types of clamp-crush techniques of robotic hepatectomy that we have performed and to assess their outcomes, safety, and feasibility. We present this article in accordance with the SUPER reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-2024-918/rc).
Preoperative preparations and requirements
Patient recruitment
A series of 58 consecutive robotic hepatectomies were performed at Iwate Medical University Hospital between June 2022 and April 2024. For comparison, 298 patients who underwent pure laparoscopic hepatectomy at Iwate Medical University Hospital between January 2014 and December 2020 were enrolled. No special selection criteria were applied. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). The Ethics Committee of Iwate Medical University Hospital approved the study (reference Nos. MH2019-120 and MH2019-122). Written informed consent was obtained from the patients for publication of this manuscript, the accompanying image and the videos. A copy of the written consent is available for review by the editorial office of this journal.
For this study, we retrospectively reviewed the prospective database of patients treated with a robotic hepatectomy at our institution.
Step-by-step description
Surgical procedure
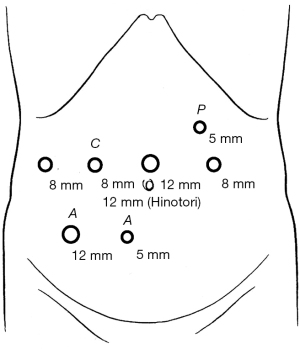
All patients were placed in the reverse Trendelenburg position (13°) and in the semi-left lateral decubitus position for lesions in the posterior sector. The surgeon stood on the right side of the patient, while the assistant and scopist stood on the patient’s left side. A 12-mm port was placed in the umbilical position. Two 8-mm robotic ports in the da Vinci-Xi surgical system (Intuitive Surgical Inc., Sunnyvale, CA, USA) and one 8-mm and one 12-mm robotic port in the HinotoriTM surgical system (Medicaroid Corporation, Kobe, Japan) were placed at the right upper quadrant and one 8-mm robotic port was placed in left upper quadrant. A 12-mm and a 5-mm trocar port were placed in the right lower quadrant for the assistant, and occasionally, a 5-mm trocar port was placed in the left upper quadrant for the Pringle maneuver (Figure 1). A carbon dioxide pneumoperitoneum was maintained at 10 mmHg.
The da Vinci-Xi surgical system robot was brought into position over the patient’s left shoulder in the HinotoriTM surgical system over the right abdomen of the patient and docked after the placement of the ports. The operator moved to the robot console to control the robotic arms. The assistant remained at the patient’s side to change robotic instruments and perform clipping, stapling, and mobilization through the assistant’s 12- and 5-mm trocar ports. To control bleeding from the liver parenchyma, the intermittent Pringle maneuver was used in more than half the patients. Additionally, low central venous pressure, low airway pressure, and low tidal volume helped control bleeding from the hepatic vein. During parenchymal transection, grasping and mobilizing is performed by the robotic fenestrated bipolar grasper on the left hand with BIPOLAR SOFT mode (effect level 4 and power limit 60 watts) in the da Vinci-Xi surgical system and with laparoscopy mode (power output is 30 watts) in the HinotoriTM surgical system. The Maryland bipolar on the right hand was the main instrument employed for the parenchymal transection using the crush-clamp technique with macro mode (power limit 60 watts) in the da Vinci-Xi surgical system and with bipolar cut mode (power output 30 watts) and Laparoscopy mode (power output 80 watts) in the HinotoriTM surgical system. An intraoperative robotic ultrasound device was employed to confirm the location of the tumor and to determine the surface cutting line of the liver.
Statistical analysis
Data were presented as mean ± standard deviation (SD), and categorical variables were described as totals and frequencies. Differences in the groups were assessed using the Mann-Whitney U test for continuous variables and the Chi-squared or Fisher’s exact test (for expected counts of <5) for categorical variables. A statistical analysis was performed using the JMP version 14.2.0 software (SAS Institute, Cary, NC, USA). Variables with P values <0.05 were considered statistically significant.
Postoperative considerations and tasks
The clinical records of 58 patients who underwent robotic hepatectomies were reviewed and analyzed. The baseline characteristics and perioperative outcomes are described in Table 1. The mean age of the 58 patients was 69.5±7.5 years. Of the patients, 45 were male, and 13 were female. In 27 cases (46.6%), a limited resection was performed, in 9 cases (15.5%), a subsegmentectomy was performed, in 15 cases (25.9%), a sectionectomy was performed, and in 7 cases (12.1%), a hemihepatectomy. In 51 cases, a hepatectomy was performed using the da Vinci-Xi surgical system, and in seven cases, the HinotoriTM surgical system was used. The mean operative time and console time were 205.9±90.5 and 99.3±67.6 min, respectively, and the mean intraoperative blood loss was 103.1±200.7 mL. There were no cases of conversion to laparotomy. The mean length of hospital stay was 10.5±7.3 days. We graded postoperative morbidity on the basis of the Clavien-Dindo classification, and grade ≥ II events were counted as postoperative complications (5). There were four cases of complications in this study. The breakdown is one case each of bleeding from inferior epigastric artery, heart failure, bile leakage, and pulmonary thromboembolism. All patients recovered without further surgical intervention, and there was no mortality in this case series (Table 1). Table 2 shows operative time, console time, parenchymal transection time, and pringle maneuver time for each surgical procedure. In comparison with laparoscopic hepatectomies, there were no significant differences in baseline characteristics other than sex and perioperative outcomes (Table 3).
Table 1
| Variables | Values (n=58) |
|---|---|
| Age (years) | 69.5±7.5 |
| Sex (male/female) | 45/13 |
| BMI (kg/m2) | 23.4±3.6 |
| Diagnosis | |
| HCC | 26 (44.8) |
| Metastasis | 28 (48.3) |
| Other (malignant) | 2 (3.4) |
| Benign | 2 (3.4) |
| Surgical procedure | |
| Limited resection | 27 (46.6) |
| Subsegmentectomy | 9 (15.5) |
| Sectionectomy | 15 (25.9) |
| Hemihepatectomy | 7 (12.1) |
| Surgical system | |
| Da Vinci-Xi | 51 (87.9) |
| HinotoriTM | 7 (12.1) |
| C-P class | |
| A | 57 (98.3) |
| B | 1 (1.7) |
| Ban’s difficulty score | 4.6±2.1 |
| Ban’s difficulty index | |
| Low | 22 (37.9) |
| Intermediate | 25 (43.1) |
| High | 11 (19.0) |
| Operative time (min) | 205.9±90.5 |
| Console time (min) | 99.3±67.6 |
| Blood loss (mL) | 103.1±200.7 |
| Blood transfusion | 2 (3.4) |
| Conversion to laparotomy | 0 (0.0) |
| Pringle maneuver | 37 (63.8) |
| Length of hospital stay (days) | 10.5±7.3 |
| Morbidity (Clavien-Dindo grade) | 4 (6.9) |
| Bleeding from inferior epigastric artery (III) | 1 (1.7) |
| Heart failure (III) | 1 (1.7) |
| Bile leakage (III) | 1 (1.7) |
| Pulmonary thromboembolism (II) | 1 (1.7) |
| Mortality | 0 (0.0) |
Values are expressed as mean ± SD, number, or number (%). BMI, body mass index; C-P, Child-Pugh; HCC, hepatocellular carcinoma; SD, standard deviation.
Table 2
| Surgical procedure | Operative time (min) | Console time (min) | Parenchymal transection time (min) | Pringle maneuver time (min) |
|---|---|---|---|---|
| Limited resection | 174.6±70.8 | 81.4±54.0 | 68.5±47.3 | 67.8±38.0 |
| Subsegmentectomy | 224.7±92.2 | 113.9±67.5 | 106.2±77.6 | 86.3±52.9 |
| Sectionectomy | 225.7±111.7 | 100.5±85.1 | 86.4±59.7 | 123.3±40.9 |
| Hemihepatectomy | 259.9±79.2 | 147.0±58.1 | 93.9±42.1 | 77.0±22.4 |
Values are expressed as mean ± SD. SD, standard deviation.
Table 3
| Variables | Rob (n=58) | Lap (n=298) | P |
|---|---|---|---|
| Age (years) | 69.5±7.5 | 66.9±11.3 | 0.16 |
| Sex (male/female) | 45/13 | 188/110 | 0.03 |
| BMI (kg/m2) | 23.4±3.6 | 23.9±3.9 | 0.50 |
| Diagnosis | 0.60 | ||
| HCC | 26 (44.8) | 156 (52.3) | |
| Metastasis | 28 (48.3) | 118 (39.6) | |
| Other (malignant) | 2 (3.4) | 16 (5.4) | |
| Benign | 2 (3.4) | 8 (2.7) | |
| Surgical procedure | 0.11 | ||
| Limited resection | 27 (46.6) | 168 (56.4) | |
| Subsegmentectomy | 9 (15.5) | 24 (8.1) | |
| Sectionectomy | 15 (25.9) | 54 (18.1) | |
| Hemihepatectomy | 7 (12.1) | 52 (17.4) | |
| C-P class | 0.77 | ||
| A | 57 (98.3) | 291 (97.7) | |
| B | 1 (1.7) | 7 (2.3) | |
| Operative time (min) | 205.9±90.5 | 200.4±83.1 | 0.91 |
| Blood loss (mL) | 103.1±200.7 | 93.9±184.7 | 0.96 |
| Conversion to laparotomy | 0 (0.0) | 2 (0.7) | 0.53 |
| Length of hospital stay (days) | 10.5±7.3 | 17.9±85.7 | 0.75 |
| Morbidity | 4 (6.9) | 31 (10.4) | 0.41 |
| Mortality | 0 (0.0) | 2 (0.7) | 0.53 |
Values are expressed as mean ± SD, number, or number (%). BMI, body mass index; C-P, Child-Pugh; HCC, hepatocellular carcinoma; SD, standard deviation.
Tips and pearls
Four clamp-crush techniques (Clamp, Peck, Open, Sweep)
Our four clamp-crush techniques are demonstrated in the video clips provided with our study’s electronic data (Video 1, Clamp; Video 2, Peck; Video 3, Open; Video 4, Sweep). At first, the four clamp-crush techniques were named Clamp-Crushing (Clamp), Clamp-Drawing (Peck), Space-Expanding (Open), and Sweeping (Sweep), but they were renamed with their current names for publication in this paper. The clamp is the most standard technique, and a hepatic parenchymal transection was performed by crushing the parenchyma and isolating the vessels and the bile ducts. The Peck is the technique in which the forceps are pulled backward without completely closing them. The name Peck derives from the fact that its movements resemble those of a bird pecking at food. In cases of liver cirrhosis, a surgeon using Clamp technique may not be able to visualize the vessels and bile ducts, and the Peck technique is effective in such cases. However, since there is no tactile sensation in robotic surgery, learning the Peck technique requires some acclimation. When using the Clamp and Peck methods, it is important to be aware of not closing the forceps completely. The Open technique is the technique in which the forceps are opened above and below the vessels and bile ducts; it is often used in laparotomies. Open can create space above and below the vessels and bile ducts. This technique is effective in cases of liver cirrhosis. The Sweep is the technique in which partially exposed vessels and bile ducts are rubbed with forceps, allowing the clear exposure of the vessels and bile ducts. Video 5 shows a procedure of combining our four clamp-crush techniques to perform liver parenchymal transection.
Discussion
Minimally invasive surgery (MIS), such as laparoscopic and robotic surgery, has become popular in the field of hepatobiliary surgery. Laparoscopic hepatectomies have gradually become the mainstream treatment due to the beneficial outcomes, including shorter hospital stays, lower morbidity, less blood loss, and lower blood transfusion rates compared with open hepatectomies (6-8). In this study, the safety and feasibility of robotic hepatectomy are comparable to laparoscopic hepatectomy. A systematic review and meta-analysis of propensity scorematched studies also reported that surgical and oncological outcomes are comparable between robotic hepatectomy and laparoscopic hepatectomy on patients with liver malignancies. Therefore, it is concluded that the benefits of applying robotic hepatectomy in patients with liver malignancies need to be further explored (9). However, the current rate of laparoscopic hepatectomies varies considerably depending on the facility (10). This is because highly difficult hepatectomies, such as major hepatectomies or posterosuperior segments, are still limited to expert laparoscopic liver surgeons (11,12). Recently, the rate of robotic hepatectomies has continued to grow because of improvements in surgical techniques and breakthroughs in surgical instrumentation, as well as due to the widespread recognition of robotic surgery (13). The advantages of a robotic hepatectomy are its high-resolution, three-dimensional imaging field of vision, motion simulation manipulator, and ability to filter the impact of human hand vibration (14), which may compensate for the inherent difficulties of the laparoscopic approach. The spread of robotic hepatectomies suggests the possibility that more surgeons may be able to perform MIS in the liver field, similar to expert laparoscopic liver surgeons.
We believe that robotic hepatectomies are more compatible with the clamp-crush method than laparoscopic hepatectomies. With robotic hepatectomies, the angle of the forceps can be controlled freely, so it is easier to perform the clamp-crush method with the cutting line of the liver than it is with a laparoscopic hepatectomy. Therefore, it is possible to perform MIS, which is closer to laparotomy than laparoscopic surgery. Furthermore, by using our four clamp-crush techniques, it is possible to clearly expose the vessels and bile ducts, even in cases of liver cirrhosis. In research in which laparoscopic hepatectomies combining a bipolar cautery and the clamp-crush method along with a robotic hepatectomy, the patients who underwent a laparoscopic hepatectomy combined with a bipolar cautery and the clamp-crush method had less blood loss and a shorter operation time than those treated with the CUSA (15). As robotic hepatectomies become more popular, there is a possibility that the clamp-crush method will receive renewed attention in the future.
A systematic review that analyzed operative parameters of robotic hepatectomies revealed the following results. The mean operative time was 277 min for all procedures, and the mean blood loss was estimated at 250 mL. The mean length of hospital stay was 6.3 days. Overall morbidity was 19%, including all complications (grades 1–4 of the Clavien-Dindo classification). There were two cases of postoperative mortality (0.2%) (16). Our results are comparable in terms of safety and feasibility, except for the length of hospital stay. In this report, the length of the hospital stay was relatively long, which may be explained by Japan’s national health insurance policies. Although the length of hospital stay is mainly determined by physicians’ clinical judgment, patients and their family members often participate in determining discharge dates (17). It may be difficult to compare the length of hospital stays in Japan with those in other countries.
In our current study, we observed four cases of postoperative complications, of which we focused on bleeding from inferior epigastric artery. Unlike the other complications, bleeding from inferior epigastric artery may be specific to robotic hepatectomies. This postoperative bleeding event was caused by 5-mm trocar port for the assistant. In robotic hepatectomies, trocar ports for the assistant must be inserted in a position that does not interfere with the robot arm. This is a position that is not used in regular laparoscopic hepatectomies. Depending on the location of the robotic port, in some cases it may be necessary to place an assistant trocar port near the inferior epigastric artery, which requires more careful port insertion than in conventional laparoscopic hepatectomies.
This study has several limitations. First, the sample size was limited. Second, we could not compare the outcomes of the clamp-crush method with other methods for performing parenchymal transections in robotic hepatectomies.
Conclusions
Our findings indicate that robotic hepatectomies are safe and feasible in high-volume specialized centers with a team experienced in laparoscopic liver surgery, even if they are in the early phases of introducing robotic hepatectomies. Intuitively, it is possible to transition from laparoscopic hepatectomy to robotic hepatectomy without stress due to the fact that the same four clamp-crush techniques that are used in laparoscopic hepatectomy, which can be done in a robotic hepatectomy. In the future, robotic hepatectomy may increase the use of MIS in the liver field.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-2024-918/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-2024-918/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-2024-918/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). The Ethics Committee of Iwate Medical University Hospital approved the study (reference Nos. MH2019-120 and MH2019-122). Written informed consent was obtained from the patients for publication of this manuscript, the accompanying image and the videos. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Giulianotti PC, Coratti A, Angelini M, et al. Robotics in general surgery: personal experience in a large community hospital. Arch Surg 2003;138:777-84. [Crossref] [PubMed]
- Takayama T, Makuuchi M, Kubota K, et al. Randomized comparison of ultrasonic vs clamp transection of the liver. Arch Surg 2001;136:922-8. [Crossref] [PubMed]
- Lesurtel M, Selzner M, Petrowsky H, et al. How should transection of the liver be performed?: a prospective randomized study in 100 consecutive patients: comparing four different transection strategies. Ann Surg 2005;242:814-22, discussion 822-3. [Crossref] [PubMed]
- Pamecha V, Gurusamy KS, Sharma D, et al. Techniques for liver parenchymal transection: a meta-analysis of randomized controlled trials. HPB (Oxford) 2009;11:275-81. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Koffron AJ, Auffenberg G, Kung R, et al. Evaluation of 300 minimally invasive liver resections at a single institution: less is more. Ann Surg 2007;246:385-92; discussion 392-4. [Crossref] [PubMed]
- Kitisin K, Packiam V, Bartlett DL, et al. A current update on the evolution of robotic liver surgery. Minerva Chir 2011;66:281-93. [PubMed]
- Cho JY, Han HS, Wakabayashi G, et al. Practical guidelines for performing laparoscopic liver resection based on the second international laparoscopic liver consensus conference. Surg Oncol 2018;27:A5-9. [Crossref] [PubMed]
- Long ZT, Li HJ, Liang H, et al. Robotic versus laparoscopic liver resection for liver malignancy: a systematic review and meta-analysis of propensity score-matched studies. Surg Endosc 2024;38:56-65. [Crossref] [PubMed]
- Viganò L, Cimino M, Aldrighetti L, et al. Multicentre evaluation of case volume in minimally invasive hepatectomy. Br J Surg 2020;107:443-51. [Crossref] [PubMed]
- Tzanis D, Shivathirthan N, Laurent A, et al. European experience of laparoscopic major hepatectomy. J Hepatobiliary Pancreat Sci 2013;20:120-4. [Crossref] [PubMed]
- Kasai M, Cipriani F, Gayet B, et al. Laparoscopic versus open major hepatectomy: a systematic review and meta-analysis of individual patient data. Surgery 2018;163:985-95. [Crossref] [PubMed]
- Zhang L, He A, Lei J, et al. Robotic hepatectomy: challenge and progression. Hepatobiliary Surg Nutr 2023;12:264-6. [Crossref] [PubMed]
- Liu R, Wakabayashi G, Kim HJ, et al. International consensus statement on robotic hepatectomy surgery in 2018. World J Gastroenterol 2019;25:1432-44. [Crossref] [PubMed]
- Yoshioka M, Shimizu T, Ueda J, et al. Safety and Feasibility of Laparoscopic Liver Resection with the Clamp-Crush Method Using the BiSect. J Nippon Med Sch 2024;91:108-13. [Crossref] [PubMed]
- Diaz-Nieto R, Vyas S, Sharma D, et al. Robotic Surgery for Malignant Liver Disease: a Systematic Review of Oncological and Surgical Outcomes. Indian J Surg Oncol 2020;11:565-72. [Crossref] [PubMed]
- Katagiri H, Nitta H, Kanno S, et al. Safety and Feasibility of Laparoscopic Parenchymal-Sparing Hepatectomy for Lesions with Proximity to Major Vessels in Posterosuperior Liver Segments 7 and 8. Cancers (Basel) 2023;15:2078. [Crossref] [PubMed]


