
Prognostic nomogram for T3–T4 primary colorectal cancer patients with perineural invasion after surgery: a Surveillance, Epidemiology, and End Results program database analysis
Highlight box
Key findings
• We constructed prognostic nomograms for predicting 3-, 5-, and 10-year overall survival (OS) and cancer-specific survival (CSS) in patients with T3–T4 primary colorectal cancer (CRC) and perineural invasion (PNI) after surgery, demonstrating high predictive accuracy and clinical utility for personalized treatment planning.
What is known and what is new?
• Elderly men with T3–T4 primary CRC and PNI after surgery experience significantly reduced OS and CSS.
• There was no statistical difference in OS and CSS between received chemotherapy or not for patients with primary T3–T4 CRC with PNI after surgery.
What is the implication, and what should change now?
• Age, sex, race, marital status, site, T stage, radiation, regional nodes positive, live metastasis, lung metastasis, tumor size, histologic type, median household income and Surveillance; Epidemiology; and End Results (SEER) summary stage were independent OS prognostic factors. Age, sex, T stage, radiation, regional nodes positive, live metastasis, lung metastasis, tumor size, histologic type and SEER summary stage were identified as independent risk factors for CSS. A novel and practical nomogram was developed. Patients were stratified into high-risk and low-risk groups, demonstrating statistically significant differences in OS. High-risk patients should receive more frequent follow-up and individualized treatment plans to improve clinical outcomes.
Introduction
Colorectal cancer (CRC) ranks as the third most prevalent malignancy and the second leading cause of cancer-related mortality globally (1). In 2020, it was estimated that there were approximately 1.93 million new cases and around 0.94 million deaths attributable to CRC worldwide, which account for 10% of the global cancer incidence and 9.4% of all cancer-related mortalities (2). CRC is a disease that is commonly encountered in the elderly population (3); however, its incidence has been increasing in patients younger than age 50 years since the mid-1990s (4). The incidence of CRC has been rapidly increasing and is projected to rise by 60% by 2030 (3). The Tumor, Node, Metastasis (TNM) staging system was established by the American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control which provides standardized, data-driven criteria for cancer reporting (5). T3–T4 stage tumors usually deeply infiltrate the muscularis propria into the pericolonic tissues and penetrate visceral peritoneum or invade adjacent organs or structures, implying a greater tumor load and deeper infiltration (5,6). As these tumors invade deeper, the prognosis is usually worse and the risk of local and distant recurrence is higher (7).
In addition to direct growth, tumor cells can grow along the nerves. Accumulating evidence indicates that the activation of neural growth within tumors, known as neoneurogenesis, plays a significant role in driving the progression of cancer (8). Perineural invasion (PNI) is a prominent characteristic of multiple solid tumors and indicates poor prognosis (9,10). Recently, as a new biologic feature, PNI has attracted more and more attention in CRC (9). PNI is defined as tumor growth in, around, and through nerves and nerve sheaths, implying that it is more aggressive (11).
A nomogram is a statistical model that combines and quantifies all proven prognostic factors in a simple graphical format (7). In recent years, several nomograms for predicting prognosis in CRC have been introduced, but none of them are specific for T3–T4 stage CRC with PNI after surgery. Therefore, we created a new nomogram that combines tumor and host factors to predict the mortality of T3–T4 stage primary CRC patients with PNI who received surgical resection. This study also has clinical value and may help improve patient survival and reduce mortality. We present this article in accordance with the TRIPOD reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-709/rc).
Methods
Patients
The data used in our study were obtained from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) 17 tumor registry database. The SEER database, known for its stringent quality control measures and requirement for a less than five percent error rate, encompasses around 28% of the United States population. It provides comprehensive data on population demographics, clinicopathological features, treatment modalities, and survival outcomes for over three million patients (12). Using SEER*Stat version 8.4.3, patients diagnosed with colon and rectum cancer between January 1, 2000 and December 31, 2019 were included in the study. Since SEER is a public domain database, patient informed consent and ethical clearance were not required to conduct this study.
The patients characteristics extracted from the SEER database included age at diagnosis, sex, race, marital status at diagnosis, year of diagnosis, tumor site, diagnostic confirmation, T stage, N stage, M stage, reason no cancer-directed surgery, radiation, chemotherapy, systemic therapy sequence with surgery, PNI status, regional nodes positive, liver metastasis, lung metastasis, tumor size, survival months, vital status, first malignant primary indicator, median household income, histological type, SEER summary stage.
The exclusion criteria were as follows: (I) patients younger than 18 years old and older than 75 years old; (II) race was unknown; (III) non-PNI; (IV) no surgery; (V) lack of positive histological confirmation; (VI) appendix and large intestine (NOS) in tumor site; (VII) marital status information such as unknown, separated, unmarried or domestic partner; (VIII) radiation recode information such as recommended, unknown if administered, refused; (IX) the codes 990–999 in CSS tumor size [2004–2015]; (X) the codes 95, 97, 98 and 99 in regional nodes positive [1988+]; (XI) lack of chemotherapy information; (XII) other incomplete clinical, pathological data and family financial situation, such as histologic type, median household income and SEER summary stage. The inclusion criteria were as follows: (I) T stage information such as T3, T4a, T4b; (II) CRC as primary tumor; (III) colon and rectum [site recode, International Classification of Diseases for Oncology (ICD-O-3)/World Health Organization (WHO) 2008]; (IV) live and lung metastasis or not. In addition, X-tile software was used to determine the best cutoff points for tumor size variable in this study. Regarding the clinical outcome, overall survival (OS) and cancer-specific survival (CSS) were chosen as the primary and second endpoints. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Statistical analysis
In the present study, OS was used as the survival primary endpoint and analyzed using the Kaplan-Meier method with log-rank test to evaluate the outcomes of T3–T4 primary CRC patients with PNI.
Using a 7:3 ratio, patients were randomly assigned to either the training cohort or the validation cohort. The study’s primary outcome indicators encompassed postoperative OS at 3-, 5-, and 10-year follow-up periods. Categorical variables were presented as numbers and percentage (n, %), and differences in variable distribution between the training and validation cohorts were assessed using the chi-square test in SPSS 22.0 (IBM, Armonk, New York, USA). Using the “survival” package in R, univariate Cox regression analyses were conducted to screen for variables significantly related to prognosis; these prognostic variables for OS were then enrolled in multivariate analyses using the Cox proportional hazards model. The assumptions underlying the Cox proportional hazards model were assessed using calibration plots and decision curve analysis (DCA) and found to be met. Nomogram integrating independent prognostic factors for 3-, 5- and 10-year postoperative OS (age, sex, race, marital status, site, T stage, radiation, regional nodes positive, live metastasis, lung metastasis, tumor size, histologic type, median household income and SEER summary stage) was created by using nomogram function of “rms” package in R software.
In addition, the Fine-Gray competing risk model was used to screen for independent risk factors affecting CSS in patients with primary CRC and PNI. A nomogram was created to predict CSS in these patients, aiming to evaluate outcomes in the presence of competing events.
The evaluation of the nomograms’ performance was conducted using a comprehensive approach. Initially, the concordance index (C-index) was utilized to evaluate the predictive performance of the nomogram. Subsequently, the area under the receiver operating characteristic (ROC) curve (AUC) was calculated to assess the nomogram’s discrimination ability. An AUC value exceeding 0.7 was considered indicative of good predictive capabilities (13). Furthermore, the nomogram was also evaluated with calibration plots in which the predicted outcomes versus the actual observed outcomes are graphically depicted. In addition, DCA was conducted to compare the clinical utility of the nomogram. All statistical analyses were executed using R software (version 4.3.3), and a two-sided P value less than 0.05 was deemed statistically significant.
Results
Baseline patient characteristics
The 7,808 patients who met the inclusion criteria were randomly allocated to either the training cohort (N=5,468) or the validation cohort (n=2,340). The demographics and clinical characteristics of patients are reported in Table 1. No statistically significant differences were observed in the basic characteristics between the two groups (all P>0.05), as outlined in Table 1. The cutoff point of tumor size was determined by X-tile (Figure 1). Specifically, 41.55% were ≤44 mm, 27.42% between 45–59 mm and 31.03% >59 mm (Table 1).
Table 1
| Variables | Total (n=7,808) | Training cohort (n=5,468) | Validation cohort (n=2,340) | P value |
|---|---|---|---|---|
| Age | 0.15 | |||
| 18–44 years | 1,032 (13.22) | 696 (12.73) | 336 (14.36) | |
| 45–64 years | 4,496 (57.58) | 3,168 (57.94) | 1,328 (56.75) | |
| 65–75 years | 2,280 (29.20) | 1,604 (29.33) | 676 (28.89) | |
| Sex | 0.75 | |||
| Female | 3,388 (43.39) | 2,379 (43.51) | 1,009 (43.12) | |
| Male | 4,420 (56.61) | 3,089 (56.49) | 1,331 (56.88) | |
| Race | 0.87 | |||
| Black | 1,067 (13.67) | 751 (13.73) | 316 (13.50) | |
| White | 5,876 (75.26) | 4,106 (75.09) | 1,770 (75.64) | |
| Other | 865 (11.08) | 611 (11.17) | 254 (10.85) | |
| Marital status | 0.80 | |||
| Single | 1,867 (23.91) | 1,298 (23.74) | 569 (24.32) | |
| Married | 4,851 (62.13) | 3,410 (62.36) | 1,441 (61.58) | |
| Divorced | 1,090 (13.96) | 760 (13.90) | 330 (14.10) | |
| Site | 0.85 | |||
| Cecum | 1,308 (16.75) | 904 (16.53) | 404 (17.26) | |
| Ascending colon | 965 (12.36) | 675 (12.34) | 290 (12.39) | |
| Hepatic flexure | 227 (2.91) | 155 (2.83) | 72 (3.08) | |
| Transverse colon | 449 (5.75) | 309 (5.65) | 140 (5.98) | |
| Splenic flexure | 252 (3.23) | 174 (3.18) | 78 (3.33) | |
| Descending colon | 474 (6.07) | 324 (5.93) | 150 (6.41) | |
| Sigmoid colon | 1,980 (25.36) | 1,403 (25.66) | 577 (24.66) | |
| Rectosigmoid junction | 891 (11.41) | 641 (11.72) | 250 (10.68) | |
| Rectum | 1,262 (16.16) | 883 (16.15) | 379 (16.20) | |
| T stage | 0.08 | |||
| T3 | 4,791 (61.36) | 3,336 (61.01) | 1,455 (62.18) | |
| T4a | 2,029 (25.99) | 1,458 (26.66) | 571 (24.40) | |
| T4b | 988 (12.65) | 674 (12.33) | 314 (13.42) | |
| Radiation | 0.95 | |||
| No | 6,433 (82.39) | 4,506 (82.41) | 1,927 (82.35) | |
| Yes | 1,375 (17.61) | 962 (17.59) | 413 (17.65) | |
| Chemotherapy | 0.30 | |||
| No | 1,882 (24.10) | 1,336 (24.43) | 546 (23.33) | |
| Yes | 5,926 (75.90) | 4,132 (75.57) | 1,794 (76.67) | |
| Systemic therapy and surgery sequence | 0.56 | |||
| No systemic therapy | 1,877 (24.04) | 1,333 (24.38) | 544 (23.25) | |
| Systemic therapy before surgery | 569 (7.29) | 385 (7.04) | 184 (7.86) | |
| Systemic therapy after surgery | 4,815 (61.67) | 3,361 (61.47) | 1,454 (62.14) | |
| Systemic therapy both before and after surgery | 519 (6.65) | 370 (6.77) | 149 (6.37) | |
| Surgery both before and after systemic therapy | 28 (0.36) | 19 (0.35) | 9 (0.38) | |
| Regional nodes positive | 0.99 | |||
| 0 | 1,802 (23.08) | 1,254 (22.93) | 548 (23.42) | |
| 1 | 1,012 (12.96) | 713 (13.04) | 299 (12.78) | |
| 2 | 825 (10.57) | 590 (10.79) | 235 (10.04) | |
| 3 | 700 (8.97) | 485 (8.87) | 215 (9.19) | |
| 4 | 576 (7.38) | 405 (7.41) | 171 (7.31) | |
| 5 | 456 (5.84) | 325 (5.94) | 131 (5.60) | |
| 6 | 390 (4.99) | 273 (4.99) | 117 (5.00) | |
| 7–8 | 613 (7.85) | 427 (7.81) | 186 (7.95) | |
| 9–10 | 424 (5.43) | 295 (5.40) | 129 (5.51) | |
| >10 | 1,010 (12.94) | 701 (12.82) | 309 (13.21) | |
| Liver metastasis | 0.77 | |||
| No | 5,840 (74.80) | 4,095 (74.89) | 1,745 (74.57) | |
| Yes | 1,968 (25.20) | 1,373 (25.11) | 595 (25.43) | |
| Lung metastasis | 0.53 | |||
| No | 7,361 (94.28) | 5,149 (94.17) | 2,212 (94.53) | |
| Yes | 447 (5.72) | 319 (5.83) | 128 (5.47) | |
| Tumor size | 0.06 | |||
| ≤44 mm | 3,244 (41.55) | 2,292 (41.92) | 952 (40.68) | |
| 45–59 mm | 2,141 (27.42) | 1,523 (27.85) | 618 (26.41) | |
| >59 mm | 2,423 (31.03) | 1,653 (30.23) | 770 (32.91) | |
| Histologic type | 0.54 | |||
| Adenocarcinoma | 7,017 (89.87) | 4,927 (90.11) | 2,090 (89.32) | |
| Mucinous adenocarcinoma | 532 (6.81) | 370 (6.77) | 162 (6.92) | |
| Signet ring cell carcinoma | 166 (2.13) | 110 (2.01) | 56 (2.39) | |
| Other | 93 (1.19) | 61 (1.12) | 32 (1.37) | |
| Median household income | 0.99 | |||
| ≤$59,999 | 2,356 (30.17) | 1,649 (30.16) | 707 (30.21) | |
| $60,000-$74,999 | 2,904 (37.19) | 2,036 (37.23) | 868 (37.09) | |
| ≥$75,000 | 2,548 (32.63) | 1,783 (32.61) | 765 (32.69) | |
| SEER summary stage | 0.46 | |||
| Localized only | 566 (7.25) | 400 (7.32) | 166 (7.09) | |
| Regional by direct extension only | 717 (9.18) | 506 (9.25) | 211 (9.02) | |
| Regional lymph nodes involved only | 1,424 (18.24) | 990 (18.11) | 434 (18.55) | |
| Regional by both direct extension and lymph node involvement | 2,246 (28.77) | 1,602 (29.30) | 644 (27.52) | |
| Distant site(s)/node(s) involved | 2,855 (36.57) | 1,970 (36.03) | 885 (37.82) | |
CRC, colorectal cancer; PNI, perineural invasion; SEER, Surveillance, Epidemiology, and End Results.

Prognostic factors affecting patient OS and CSS
Based on the analysis of univariate data, age, sex, race, marital status, site, T stage, radiation, chemotherapy, systemic therapy and surgery sequence, regional nodes positive, live metastasis, lung metastasis, tumor size, histologic type, median household income and SEER summary stage, were found to be significant prognostic factors for OS in T3–T4 primary CRC patients with PNI. The values were plotted as Kaplan-Meier survival curves and compared using log-rank test (Figure 2). To further identify the independent prognostic factors for OS in patients with T3–T4 primary CRC with PNI, all risk factors for OS identified by multivariate analysis, which included the following: (I) aged 45–64 years and 65–75 years; (II) male gender; (III) White race and other race; (IV) married; (V) site: splenic flexure, sigmoid colon and rectosigmoid junction; (VI) T4a and T4b stage; (VII) received radiation; (VIII) regional nodes positive: 2, 3, 4, 5, 6, 7–8, 9–10 and >10; (IX) live metastasis; (X) lung metastasis; (XI) tumor size >59 mm; (XII) histologic type: mucinous adenocarcinoma, signet ring cell carcinoma and other type; (XIII) median household income >$75,000; (XIV) SEER summary stage: regional by direct extension only, regional lymph nodes involved only, regional by both direct extension and lymph node involvement and distant site(s)/node(s) involved (all P<0.05) (Table 2).

Table 2
| Variables | Alive/death | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |||
| Age | ||||||
| 18–44 years | 449/583 | Reference | Reference | |||
| 45–64 years | 1,706/2,790 | 1.167 (1.067–1.276) | <0.001 | 1.249 (1.141–1.369) | <0.001 | |
| 65–75 years | 738/1,542 | 1.379 (1.253–1.517) | <0.001 | 1.533 (1.386–1.696) | <0.001 | |
| Sex | ||||||
| Female | 1,371/2,017 | Reference | Reference | |||
| Male | 1,522/2,898 | 1.151 (1.087–1.218) | <0.001 | 1.201 (1.133–1.273) | <0.001 | |
| Race | ||||||
| Black | 315/752 | Reference | Reference | |||
| White | 2,213/3,663 | 0.805 (0.745–0.871) | <0.001 | 0.825 (0.76–0.895) | <0.001 | |
| Other | 365/500 | 0.729 (0.651–0.816) | <0.001 | 0.77 (0.684–0.867) | <0.001 | |
| Marital status | ||||||
| Single | 616/1,251 | Reference | Reference | |||
| Married | 1,914/2,937 | 0.808 (0.756–0.863) | <0.001 | 0.835 (0.779–0.894) | <0.001 | |
| Divorced | 363/727 | 0.974 (0.889–1.067) | 0.57 | 1.023 (0.932–1.123) | 0.63 | |
| Site | ||||||
| Cecum | 390/918 | Reference | Reference | |||
| Ascending colon | 361/604 | 0.759 (0.684–0.841) | <0.001 | 0.92 (0.829–1.021) | 0.12 | |
| Hepatic flexure | 89/138 | 0.740 (0.619–0.886) | <0.001 | 1.039 (0.867–1.245) | 0.67 | |
| Transverse colon | 166/283 | 0.737 (0.645–0.842) | <0.001 | 0.905 (0.791–1.036) | 0.15 | |
| Splenic flexure | 101/151 | 0.698 (0.587–0.829) | <0.001 | 0.761 (0.639–0.906) | 0.002 | |
| Descending colon | 187/287 | 0.681 (0.596–0.777) | <0.001 | 0.901 (0.787–1.031) | 0.13 | |
| Sigmoid colon | 767/1,213 | 0.691 (0.634–0.753) | <0.001 | 0.818 (0.749–0.894) | <0.001 | |
| Rectosigmoid junction | 335/556 | 0.687 (0.619–0.764) | <0.001 | 0.796 (0.713–0.888) | <0.001 | |
| Rectum | 497/765 | 0.641 (0.582–0.706) | <0.001 | 0.993 (0.876–1.126) | 0.92 | |
| T stage | ||||||
| T3 | 2,163/2,628 | Reference | Reference | |||
| T4a | 533/1,496 | 1.825 (1.712–1.945) | <0.001 | 1.474 (1.372–1.583) | <0.001 | |
| T4b | 197/791 | 2.209 (2.039–2.392) | <0.001 | 1.5 (1.375–1.644) | <0.001 | |
| Radiation | ||||||
| No | 2,333/4,100 | Reference | Reference | |||
| Yes | 560/815 | 0.789 (0.732–0.851) | <0.001 | 1.171 (1.052–1.304) | 0.002 | |
| Chemotherapy | ||||||
| No | 647/1,235 | Reference | Reference | |||
| Yes | 2,246/3,680 | 0.751 (0.704–0.801) | <0.001 | 1.632 (0.407–6.541) | 0.49 | |
| Systemic therapy and surgery sequence | ||||||
| No systemic therapy | 644/1,233 | Reference | Reference | |||
| Systemic therapy before surgery | 197/372 | 0.809 (0.72–0.908) | <0.001 | 0.308 (0.076–1.241) | 0.10 | |
| Systemic therapy after surgery | 1,847/2,968 | 0.748 (0.7–0.799) | <0.001 | 0.253 (0.063–1.017) | 0.053 | |
| Systemic therapy both before and after surgery | 195/324 | 0.7 (0.62–0.792) | <0.001 | 0.249 (0.061–1.005) | 0.051 | |
| Surgery both before and after systemic therapy | 2,025/10 | 0.764 (0.48–1.217) | 0.26 | 0.294 (0.068–1.276) | 0.10 | |
| Regional nodes positive | ||||||
| 0 | 972/890 | Reference | Reference | |||
| 1 | 459/553 | 1.273 (1.143–1.417) | <0.001 | 1.015 (0.885–1.164) | 0.83 | |
| 2 | 322/503 | 1.527 (1.367–1.706) | <0.001 | 1.216 (1.058–1.399) | 0.006 | |
| 3 | 257/443 | 1.629 (1.451–1.828) | <0.001 | 1.314 (1.139–1.517) | <0.001 | |
| 4 | 197/379 | 1.746 (1.546–1.971) | <0.001 | 1.372 (1.183–1.591) | <0.001 | |
| 5 | 150/306 | 1.861 (1.633–2.122) | <0.001 | 1.446 (1.236–1.692) | <0.001 | |
| 6 | 113/277 | 2.062 (1.8–2.363) | <0.001 | 1.518 (1.292–1.782) | <0.001 | |
| 7–8 | 175/438 | 2.161 (1.925–2.427) | <0.001 | 1.554 (1.345–1.796) | <0.001 | |
| 9–10 | 94/330 | 2.706 (2.381–3.075) | <0.001 | 1.836 (1.574–2.142) | <0.001 | |
| >10 | 154/856 | 3.544 (3.219–3.902) | <0.001 | 2.446 (2.147–2.787) | <0.001 | |
| Liver metastasis | ||||||
| No | 2,696/3,144 | Reference | Reference | |||
| Yes | 197/1,771 | 3.142 (2.96–3.336) | <0.001 | 1.568 (1.431–1.718) | <0.001 | |
| Lung metastasis | ||||||
| No | 2,863/4,498 | Reference | Reference | |||
| Yes | 30/417 | 2.769 (2.502–3.064) | <0.001 | 1.411 (1.268–1.57) | <0.001 | |
| Tumor size | ||||||
| ≤44 mm | 1,391/1,853 | Reference | Reference | |||
| 45–59 mm | 767/1,374 | 1.276 (1.19–1.369) | <0.001 | 1.071 (0.998–1.149) | 0.057 | |
| >59 mm | 735/1,688 | 1.587 (1.485–1.695) | <0.001 | 1.207 (1.126–1.293) | <0.001 | |
| Histologic type | ||||||
| Adenocarcinoma | 2,688/4,329 | Reference | Reference | |||
| Mucinous adenocarcinoma | 158/374 | 1.322 (1.19–1.47) | <0.001 | 1.182 (1.061–1.317) | 0.002 | |
| Signet ring cell carcinoma | 23/143 | 2.429 (2.056–2.871) | <0.001 | 2.004 (1.685–2.383) | <0.001 | |
| Other | 24/69 | 1.925 (1.518–2.442) | <0.001 | 1.943 (1.527–2.473) | <0.001 | |
| Median household income | ||||||
| ≤$59,999 | 810/1,546 | Reference | Reference | |||
| $60,000–$74,999 | 1,062/1,842 | 0.946 (0.884–1.012) | 0.11 | 0.96 (0.896–1.028) | 0.25 | |
| ≥$75,000 | 1,021/1,527 | 0.878 (0.818–0.942) | <0.001 | 0.893 (0.83–0.961) | 0.003 | |
| SEER summary stage | ||||||
| Localized only | 393/173 | Reference | Reference | |||
| Regional by direct extension only | 413/304 | 1.495 (1.24–1.802) | <0.001 | 1.463 (1.21–1.768) | <0.001 | |
| Regional lymph nodes involved only | 767/657 | 1.682 (1.422–1.989) | <0.001 | 1.932 (1.577–2.367) | <0.001 | |
| Regional by both direct extension and lymph node involvement | 985/1,261 | 2.312 (1.973–2.711) | <0.001 | 2.125 (1.744–2.591) | <0.001 | |
| Distant site(s)/node(s) involved | 335/2,520 | 6.486 (5.555–7.572) | <0.001 | 4.648 (3.79–5.699) | <0.001 | |
CI, confidence interval; CRC, colorectal cancer; HR, hazard ratio; OS, overall survival; PNI, perineural invasion; SEER, Surveillance, Epidemiology, and End Results.
In the analysis of prognostic factors influencing CSS in patients, mortality was treated as a competing risk factor using the Fine-Gray competing risk regression model. A total of 2,893 patients survived, while 4,915 died; 4,324 died due to disease-specific causes and 591 died due to other causes. Independent prognostic factors were identified using multivariate Fine-Gray analysis and subsequently included in the multivariate independent prognostic analysis. The multivariate independent prognostic analysis showed that age, sex, T stage, radiation, regional nodes positive, live metastasis, lung metastasis, tumor size, histologic type and SEER summary stage were identified as independent risk factors for CSS [all hazard ratio (HR) >1, P<0.05] (Table 3).
Table 3
| Variables | Alive | Cancer-specific death | Other-cause death | HR (95% CI) | P value |
|---|---|---|---|---|---|
| Age | |||||
| 18–44 years | 449 | 551 | 32 | Reference | |
| 45–64 years | 1,706 | 2,495 | 295 | 1.135 (1.034–1.247) | 0.008 |
| 65–75 years | 738 | 1,278 | 264 | 1.271 (1.142–1.416) | <0.001 |
| Sex | |||||
| Female | 1,371 | 1,805 | 212 | Reference | |
| Male | 1,522 | 2,519 | 379 | 1.139 (1.067–1.216) | <0.001 |
| Race | |||||
| Black | 315 | 645 | 107 | Reference | |
| White | 2,213 | 3,236 | 427 | 0.873 (0.793–0.962) | 0.006 |
| Other | 365 | 443 | 57 | 0.841 (0.735–0.963) | 0.01 |
| Marital status | |||||
| Single | 616 | 1,089 | 162 | Reference | |
| Married | 1,914 | 2,618 | 319 | 0.921 (0.849–0.998) | 0.046 |
| Divorced | 363 | 617 | 110 | 1.03 (0.921–1.152) | 0.60 |
| Site | |||||
| Cecum | 390 | 833 | 85 | Reference | |
| Ascending colon | 361 | 525 | 79 | 0.947 (0.835–1.075) | 0.41 |
| Hepatic flexure | 89 | 124 | 14 | 1.121 (0.914–1.374) | 0.27 |
| Transverse colon | 166 | 238 | 45 | 0.821 (0.69–0.963) | 0.03 |
| Splenic flexure | 101 | 133 | 18 | 0.791 (0.65–0.963) | 0.02 |
| Descending colon | 187 | 243 | 44 | 0.877 (0.754–1.02) | 0.09 |
| Sigmoid colon | 767 | 1,063 | 150 | 0.832 (0.75–0.924) | <0.001 |
| Rectosigmoid junction | 335 | 490 | 66 | 0.805 (0.708–0.915) | <0.001 |
| Rectum | 497 | 675 | 90 | 0.961 (0.833–1.12) | 0.59 |
| T stage | |||||
| T3 | 2,163 | 2,219 | 409 | Reference | |
| T4a | 533 | 1,376 | 120 | 1.463 (1.352–1.585) | <0.001 |
| T4b | 197 | 729 | 62 | 1.482 (1.33–1.647) | <0.001 |
| Radiation | |||||
| No | 2,333 | 3,601 | 499 | Reference | |
| Yes | 560 | 723 | 92 | 1.211 (1.078–1.36) | 0.001 |
| Chemotherapy | |||||
| No | 647 | 999 | 236 | Reference | |
| Yes | 2,246 | 3,325 | 355 | 1.484 (0.55–4.01) | 0.44 |
| Systemic therapy and surgery sequence | |||||
| No systemic therapy | 644 | 997 | 236 | Reference | |
| Systemic therapy before surgery | 197 | 328 | 44 | 0.433 (0.159–1.181) | 0.10 |
| Systemic therapy after surgery | 1,847 | 2,679 | 289 | 0.37 (0.137–1.001) | 0.050 |
| Systemic therapy both before and after surgery | 195 | 302 | 22 | 0.379 (0.139–1.035) | 0.058 |
| Surgery both before and after systemic therapy | 10 | 18 | 0 | 0.482 (0.163–1.426) | 0.19 |
| Regional nodes positive | |||||
| 0 | 972 | 651 | 179 | Reference | |
| 1 | 459 | 468 | 85 | 1.053 (0.908–1.222) | 0.49 |
| 2 | 322 | 440 | 63 | 1.273 (1.096–1.479) | 0.001 |
| 3 | 257 | 384 | 59 | 1.321 (1.128–1.548) | <0.001 |
| 4 | 197 | 345 | 34 | 1.496 (1.28–1.75) | <0.001 |
| 5 | 150 | 275 | 31 | 1.433 (1.206–1.701) | <0.001 |
| 6 | 113 | 252 | 25 | 1.57 (1.312–1.879) | <0.001 |
| 7–8 | 175 | 395 | 43 | 1.558 (1.326–1.83) | <0.001 |
| 9–10 | 94 | 309 | 21 | 1.839 (1.532–2.08) | <0.001 |
| >10 | 154 | 805 | 51 | 2.502 (2.161–2.896) | <0.001 |
| Liver metastasis | |||||
| No | 2,696 | 2,636 | 508 | Reference | |
| Yes | 197 | 1,688 | 83 | 1.584 (1.424–1.761) | <0.001 |
| Lung metastasis | |||||
| No | 2,863 | 3,928 | 570 | Reference | |
| Yes | 30// | 396 | 21 | 1.356 (1.203–1.528) | <0.001 |
| Tumor size | |||||
| ≤44 mm | 1,391 | 1,612 | 241 | Reference | |
| 45–59 mm | 767 | 1,214 | 160 | 1.056 (0.977–1.143) | 0.17 |
| >59 mm | 735 | 1,498 | 190 | 1.151 (1.064–1.244) | <0.001 |
| Histologic type | |||||
| Adenocarcinoma | 2,688 | 3,795 | 534 | Reference | |
| Mucinous adenocarcinoma | 158 | 332 | 42 | 1.208 (1.067–1.368) | 0.002 |
| Signet ring cell carcinoma | 23 | 131 | 12 | 1.744 (1.4–2.173) | <0.001 |
| Other | 24 | 66 | 3 | 2.068 (1.49–2.871) | <0.001 |
| Median household income | |||||
| ≤$59,999 | 810 | 1,332 | 214 | Reference | |
| $60,000–$74,999 | 1,062 | 1,623 | 219 | 1.008 (0.931–1.091) | 0.84 |
| ≥$75,000 | 1,021 | 1,369 | 158 | 0.939 (0.864–1.02) | 0.14 |
| SEER summary stage | |||||
| Localized only | 393 | 105 | 68 | Reference | |
| Regional by direct extension only | 413 | 232 | 72 | 1.762 (1.393–2.229) | <0.001 |
| Regional lymph nodes involved only | 767 | 516 | 141 | 2.053 (1.605–2.626) | <0.001 |
| Regional by both direct extension and lymph node involvement | 985 | 1,091 | 170 | 2.468 (1.941–3.139) | <0.001 |
| Distant site(s)/node(s) involved | 335 | 2,380 | 140 | 5.197 (4.048–6.673) | <0.001 |
CI, confidence interval; CRC, colorectal cancer; CSS, cancer-specific survival; HR, hazard ratio; PNI, perineural invasion; SEER, Surveillance, Epidemiology, and End Results.
Construction of the nomogram
Interestingly, age, sex, race, marital status, site, T stage, radiation, chemotherapy, systemic therapy and surgery sequence, regional nodes positive, live metastasis, lung metastasis, tumor size, histologic type, median household income and SEER summary stage were all displayed significant difference in univariate OS analysis (Table 2). Next, age, sex, race, marital status, site, T stage, radiation, regional nodes positive, live metastasis, lung metastasis, tumor size, histologic type, median household income and SEER summary stage were significantly identified in OS multivariate analysis (Table 2). The multivariate independent prognostic analysis showed age, sex, T stage, radiation, regional nodes positive, live metastasis, lung metastasis, tumor size, histologic type and SEER summary stage were identified as independent risk factors for CSS. Nomogram can be used to evaluate the 3-, 5-, and 10-year OS and CSS of patients with primary T3–T4 stage CRC with PNI. Then a predictive nomogram model was established based on the factors identified by multivariate analysis (Figures 3,4). The risk score for each variable was derived from the nomogram, and their summation yielded a total score, which was used to predict OS and CSS for individual patients at 3, 5, and 10 years (Figures 3,4).


Nomogram validation
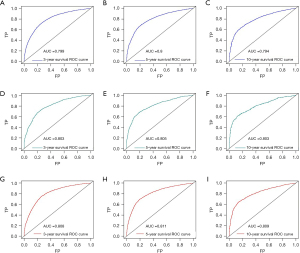
The nomogram’s utility was assessed using C-index, ROC curve, calibration plots and DCA. The C-index of OS nomogram was 0.7422 in training cohort while 0.7428 in validation cohort, and was 0.7208 in CSS dataset. We evaluated the discriminatory ability of the nomogram by the ROC curve. ROC curve analysis showed that the AUC of the 3-, 5-, and 10-year OS of the nomogram were 0.799, 0.8 and 0.794 in the training cohort, and 0.803, 0.805 and 0.803 in validation cohort, and 0.808, 0.811 and 0.809 in CSS cohort, respectively (Figure 5). Next, the nomogram’s calibration was assessed through calibration plots, revealing that the predicted and observed probabilities of T3–T4 primary CRC with PNI after surgery were consistent between training, validation and CSS cohorts (Figure 6). Furthermore, the DCA analyzed the nomogram’s clinical usefulness in the training, validation and CSS cohort, revealing significant positive net benefits (Figure 7).


Discussion
A large population-based study reveals a strong correlation between the demographic characteristics, clinicopathological and therapeutic factors, and survival outcomes of patients with primary CRC with PNI. Over the past decade, global trends have shown a rise in the incidence and mortality rates of CRC, with both rates also increasing in our country (2,3,14). CRC typically presents with few or no clinical symptoms in its early stages, leading to many patients being diagnosed only once the disease has reached a locally advanced or advanced stage. Locally advanced CRC is characterized by T3 tumors that invade 5 mm or more beyond the muscularis propria, T4 tumors with direct invasion into nearby structures, or significant regional lymph node involvement, all occurring without distant metastases (15,16). However, advanced stages primarily involve both local spread and distant metastasis. Additionally, PNI, a route for metastatic spread in cancers such as CRC, is linked to a poor prognosis (17). Thus, exploring the prognostic indicators of primary T3–T4 stage CRC with PNI after surgery has significant clinical importance.
A nomogram is a tool designed to predict OS probabilities for individual patients, assisting clinicians in developing personalized treatment plans. Accurate survival predictions are essential for selecting appropriate clinical strategies. Through univariate and multivariate analyses, fourteen independent prognostic factors were identified, and a predictive nomogram was subsequently developed using these factors.
In our present study, we found that as age increases, patients with primary CRC at T3–T4 stages with PNI after surgery have a lower OS and a higher mortality rate. This finding is consistent with previous studies, which have also identified age as an independent prognostic factor for many cancer patients, including those with locally advanced or advanced CRC (18-20). Our research also indicates that men are at a higher risk of disease and have a lower OS and CSS rate compared to women, consistent with prior studies (20,21). Variations in diet and lifestyle, combined with the protective role of estrogens in females against CRC (22,23), may explain why female CRC patients tend to have a survival advantage over male CRC patients (24). And, in this study, the OS of White and other racial was significantly higher than that of Black individuals, with both groups having a lower risk of death compared to Black individuals. Meanwhile, our study found that PNI in primary CRC with larger tumors and distant organ metastasis is an independent prognostic factor for both OS and CSS, and is associated with lower OS and CSS. This could be attributed to the propensity of larger tumors to penetrate the serosal layer, and when accompanied by distant metastasis, it often results in advanced cancer, missing the optimal surgical window, and leading to a poorer prognosis. In this study, the X-tile software was used to determine the optimal tumor size cutoffs of 44 and 59 mm for predicting the prognosis of patients with T3–T4 primary CRC with PNI. Based on these cutoffs, patients were categorized into low-risk, medium-risk, and high-risk groups, which could provide valuable reference for future patient screening.
The result of this study indicate that tumor metastasis is an independent prognostic factor that affects OS and CSS in patients with primary T3–T4 stage CRC with PNI. Furthermore, patients with liver metastasis have the poorest prognoses, which is consistent with the results from prior study on advanced CRC (20). This may be due to the fact that patients with systemic metastases often miss the optimal window for curative surgery and have a high tumor burden, making chemotherapy less effective and leading to significantly lower survival rates. Previous research has demonstrated that surgery targeting metastases can extend survival and enhance patient outcomes. Consequently, for patients with CRC who have organ metastases and are in a physical condition suitable for surgery, and where the remaining metastases are functioning adequately, surgical intervention is advised to increase their chances of survival (20). Our research also found that compared to left-sided colon cancer, right-sided colon cancer is associated with lower OS and CSS. And right-sided colon cancer is an independent prognostic risk factor for both OS and CSS. However, scientists have proposed that right-sided (proximal) colon cancer is a more aggressive form of tumor compared to left-sided (distal) colon cancer, which is consistent with our study findings (25). Interestingly, the greater the number of positive regional lymph nodes, the worse the patient’s prognosis, and the lower the OS and CSS. The observed differences may be due to the possibility that the number of positive regional lymph nodes better reflects the severity and progression of the disease compared to the N stage. Nowadays, radiation therapy is widely used in the treatment of advanced rectal tumors. It works by damaging various biomolecules, such as proteins and lipids, with DNA being the most critical target. However, radiation therapy can also lead to the development of radioresistance (26). These factors may exacerbate the condition of patients with advanced tumors. In our study, we also found that the risk of death increased by 22.4% in CRC patients who received postoperative radiation therapy.
Meanwhile, we found that, compared to singles, married patients have a significantly lower risk of death for both OS and CSS in our study. It is possible that diminished psychosocial support and elevated psychological stress may compromise immune function, potentially accelerating tumor progression and increasing the risk of death (27,28). Similarly, Johansen et al. (29) and Wang et al. (30) reported that the survival time of patients with colon cancer who were married at the time of diagnosis was significantly longer than that of patients who had never been married, which is consistent with our study findings. Our study also found that compared to adenocarcinoma, the OS and CSS of patients with mucinous adenocarcinoma, signet ring cell carcinoma, and other tumor types were significantly lower. The main reasons for the poor prognosis of mucinous adenocarcinoma and signet ring cell carcinoma are the advanced tumor stage and poor differentiation at the time of diagnosis, which are associated with a higher risk of tumor recurrence and tumor-related mortality (31,32). Finally, our study results indicate that patients with higher household incomes have improved OS. This is likely because these patients have the financial means to access superior treatment options.
Several limitations should be acknowledged in our study. First, the SEER database does not comprehensively cover all factors that influence CRC prognosis, such as smoking history, alcohol consumption, dietary habits, family history, and tumor markers, which may introduce bias into the results. Second, the SEER database lacks detailed information on patients’ radiotherapy and chemotherapy, which may be influenced by subjective patient preferences. Third, due to incomplete clinical information for some primary T3–T4 CRC patients with PNI, we excluded them from the study based on inclusion and exclusion criteria, potentially introducing bias. Fourth, the study utilized univariate analysis and forward selection, which have inherent limitations. Future research will incorporate existing knowledge and methods, such as DAG, and introduce the cumulative incidence function to present the absolute risk of events, thus avoiding excessive reliance on HR. Fifth, the model in this study is applicable to the SEER database sample, and future studies will include multi-center research in China to broaden the model’s applicability. Despite these limitations, the strength of this study lies in its extensive demographic data, making it the first to analyze the dataset of postoperative primary T3–T4 CRC patients with PNI.
Conclusions
This study focused on constructing and validating a nomogram designed to predict OS and CSS in patients with primary T3–T4 stage CRC with PNI after surgery. The nomogram exhibited robust reliability and practical clinical utility, helping clinicians assess patients’ risk factors and formulate optimal personalized treatment strategies. However, future large-scale prospective clinical trials are needed for external validation to refine these predictive models and ensure they provide appropriate and accurate tools for patient risk assessment.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-709/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-709/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-709/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Baidoun F, Elshiwy K, Elkeraie Y, et al. Colorectal Cancer Epidemiology: Recent Trends and Impact on Outcomes. Curr Drug Targets 2021;22:998-1009. [Crossref] [PubMed]
- Lun W, Luo C. Second primary colorectal cancer in adults: a SEER analysis of incidence and outcomes. BMC Gastroenterol 2023;23:253. [Crossref] [PubMed]
- Kaya T, Dursun A. Can Lymphovascular and Perineural Invasion be Additional Staging Criteria in Colorectal Cancer? J Coll Physicians Surg Pak 2021;31:657-62. [Crossref] [PubMed]
- Siegel RL, Jakubowski CD, Fedewa SA, et al. Colorectal Cancer in the Young: Epidemiology, Prevention, Management. Am Soc Clin Oncol Educ Book 2020;40:1-14. [Crossref] [PubMed]
- Yantiss RK. Persistent Problems in Colorectal Cancer Reporting. Surg Pathol Clin 2017;10:961-76. [Crossref] [PubMed]
- Zeng H, Xue X, Chen D, et al. Conditional survival analysis and real-time prognosis prediction in stage III T3-T4 colon cancer patients after surgical resection: a SEER database analysis. Int J Colorectal Dis 2024;39:54. [Crossref] [PubMed]
- Li C, Pei Q, Zhu H, et al. Survival nomograms for stage III colorectal cancer. Medicine (Baltimore) 2018;97:e13239. [Crossref] [PubMed]
- Hutchings C, Phillips JA, Djamgoz MBA. Nerve input to tumours: Pathophysiological consequences of a dynamic relationship. Biochim Biophys Acta Rev Cancer 2020;1874:188411. [Crossref] [PubMed]
- Hu G, Li L, Hu K. Clinical implications of perineural invasion in patients with colorectal cancer. Medicine (Baltimore) 2020;99:e19860. [Crossref] [PubMed]
- Yang YH, Liu JB, Gui Y, et al. Relationship between autophagy and perineural invasion, clinicopathological features, and prognosis in pancreatic cancer. World J Gastroenterol 2017;23:7232-41. [Crossref] [PubMed]
- Qin L, Heng Y, Deng S, et al. Perineural invasion affects prognosis of patients undergoing colorectal cancer surgery: a propensity score matching analysis. BMC Cancer 2023;23:452. [Crossref] [PubMed]
- Schroeder MC, Rastogi P, Geyer CE Jr, et al. Early and Locally Advanced Metaplastic Breast Cancer: Presentation and Survival by Receptor Status in Surveillance, Epidemiology, and End Results (SEER) 2010-2014. Oncologist 2018;23:481-8. [Crossref] [PubMed]
- Liu Y, Wang J, Li L, et al. AC010973.2 promotes cell proliferation and is one of six stemness-related genes that predict overall survival of renal clear cell carcinoma. Sci Rep 2022;12:4272. [Crossref] [PubMed]
- Benitez Majano S, Di Girolamo C, Rachet B, et al. Surgical treatment and survival from colorectal cancer in Denmark, England, Norway, and Sweden: a population-based study. Lancet Oncol 2019;20:74-87. [Crossref] [PubMed]
- Gosavi R, Chia C, Michael M, et al. Neoadjuvant chemotherapy in locally advanced colon cancer: a systematic review and meta-analysis. Int J Colorectal Dis 2021;36:2063-70. [Crossref] [PubMed]
- Eisenberg SB, Kraybill WG, Lopez MJ. Long-term results of surgical resection of locally advanced colorectal carcinoma. Surgery 1990;108:779-85; discussion 785-6. [PubMed]
- Knijn N, Mogk SC, Teerenstra S, et al. Perineural Invasion is a Strong Prognostic Factor in Colorectal Cancer: A Systematic Review. Am J Surg Pathol 2016;40:103-12. [Crossref] [PubMed]
- Kong X, Li J, Cai Y, et al. A modified TNM staging system for non-metastatic colorectal cancer based on nomogram analysis of SEER database. BMC Cancer 2018;18:50. [Crossref] [PubMed]
- Zhang ZY, Luo QF, Yin XW, et al. Nomograms to predict survival after colorectal cancer resection without preoperative therapy. BMC Cancer 2016;16:658. [Crossref] [PubMed]
- Tang X, Hu N, Huang S, et al. Prognostic nomogram for colorectal cancer patients with multi-organ metastases: a Surveillance, Epidemiology, and End Results program database analysis. J Cancer Res Clin Oncol 2023;149:12131-43. [Crossref] [PubMed]
- Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet 2014;383:1490-502. [Crossref] [PubMed]
- Jung KW, Park S, Shin A, et al. Do female cancer patients display better survival rates compared with males? Analysis of the Korean National Registry data, 2005-2009. PLoS One 2012;7:e52457. [Crossref] [PubMed]
- Hendifar A, Yang D, Lenz F, et al. Gender disparities in metastatic colorectal cancer survival. Clin Cancer Res 2009;15:6391-7. [Crossref] [PubMed]
- Lee SHF, Abdul Rahman H, Abidin N, et al. Survival of colorectal cancer patients in Brunei Darussalam: comparison between 2002-09 and 2010-17. BMC Cancer 2021;21:477. [Crossref] [PubMed]
- Kim SE, Paik HY, Yoon H, et al. Sex- and gender-specific disparities in colorectal cancer risk. World J Gastroenterol 2015;21:5167-75. [Crossref] [PubMed]
- Tessmann JW, Rocha MR, Morgado-Díaz JA. Mechanisms of radioresistance and the underlying signaling pathways in colorectal cancer cells. J Cell Biochem 2023;124:31-45. [Crossref] [PubMed]
- Garssen B, Goodkin K. On the role of immunological factors as mediators between psychosocial factors and cancer progression. Psychiatry Res 1999;85:51-61. [Crossref] [PubMed]
- Moreno-Smith M, Lutgendorf SK, Sood AK. Impact of stress on cancer metastasis. Future Oncol 2010;6:1863-81. [Crossref] [PubMed]
- Johansen C, Schou G, Soll-Johanning H, et al. Influence of marital status on survival from colon and rectal cancer in Denmark. Br J Cancer 1996;74:985-8. [Crossref] [PubMed]
- Wang L, Wilson SE, Stewart DB, et al. Marital status and colon cancer outcomes in US Surveillance, Epidemiology and End Results registries: does marriage affect cancer survival by gender and stage? Cancer Epidemiol 2011;35:417-22. [Crossref] [PubMed]
- Nitsche U, Friess H, Agha A, et al. Prognosis of mucinous and signet-ring cell colorectal cancer in a population-based cohort. J Cancer Res Clin Oncol 2016;142:2357-66. [Crossref] [PubMed]
- Zhuang Y, Wang H, Jiang D, et al. Multi gene mutation signatures in colorectal cancer patients: predict for the diagnosis, pathological classification, staging and prognosis. BMC Cancer 2021;21:380. [Crossref] [PubMed]


