
Autoimmune enteropathy associated with T cell large granular lymphocytic leukemia in a patient with BACH2 mutation: a case report
Highlight box
Key findings
• Autoimmune enteropathy is associated with T cell large granular lymphocytic leukemia (T-LGL) with non-STAT mutation.
What is known and what is new?
• T-LGL is a rare indolent lymphoproliferative disorder.
• Reports an association between autoimmune enteropathy and T-LGL.
What is the implication, and what should change now?
• The first case of T-LGL-associated autoimmune enteropathy in a patient with a germline BACH2 mutation. Further study of the role of Bach2 in pathogenesis of T-LGL and autoimmune disorders is warranted.
Introduction
T cell large granular lymphocytic leukemia (T-LGL) is a rare lymphoproliferative disorder characterized by a chronic clonal proliferation of mature cytotoxic T cells, involving bone marrow, spleen, liver and peripheral blood. T-LGL mainly occurs in elderly individuals with a median age of 60 years. There is no difference in incidence between males and females. It commonly presents with cytopenia and is associated with autoimmune disorders including rheumatoid arthritis, systemic lupus erythematosus (SLE), Sjogren syndrome, Hashimoto’s disease, coagulopathy, vasculitis and myositis (1,2). Rare gastrointestinal involvements have been reported including celiac disease, inflammatory bowel disease (IBD), and autoimmune hepatitis (1,3-7). Only one case report has described a clear association between autoimmune enteropathy and T-LGL. However, no histological features were shown in that case (7). The most common type is CD8 positive T cells coexpressing aberrant NK cell-associated markers, like CD16 and CD57. Pathogenesis of the tumor is believed to be caused by clonal expansion of large granular T cells induced by chronic antigenic stimulation, plus constitutively upregulated survival signaling and suppressed apoptotic signaling. Somatic mutations of STAT3 and STAT5b have been found in half of patients (1,8). Here we report an extremely rare case of T-LGL with autoimmune disorders and non-STAT gene mutations. We present this case in accordance with the CARE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-804/rc).
Case presentation
A 36-year-old female with a past medical history of chronic pulmonary hypertension, type I diabetes mellitus, pancreatic insufficiency, pure red cell aplasia, pancytopenia, refractory T-LGL (diagnosed 15 years ago), recurrent infection and bacteremia. She was diagnosed with IgA deficiency and celiac disease 3 years ago, which was confirmed by increased anti-tissue transglutaminase IgG antibody (tTG-IgG). She was found to harbor an HLA-DQ8 allele. In the past, the T-LGL had been treated with prednisone, dacluzimab, cyclosporine, oral methotrexate, cyclophosphamide, pentostatin, anti-thymocyte globulin, and IVIG. The patient was transfusion dependent and on treatments with tacrolimus, danazol and Filgrastim. She was also on multiple rounds of antibiotics for chronic sinusitis.
She presented on admission for evaluation of acute and chronic watery non-bloody diarrhea. The chronic diarrhea started in 2019 and became progressively worse. She denied abdominal pain, nausea or vomiting. The patient was not in acute distress. The abdomen was flat and soft. There was no mass, tenderness or guarding. Bowel sounds were normal. Lab tests showed: WBC 1.9 (4×103–10×103/µL), RBC 2.6 (3.9×106–5.2×106/µL), hemoglobin 6.9 (11.2–15.7 g/dL), and platelets 60 (160×103–370×103/µL). The patient’s IgG level was 1,078 mg/dL and IgA was <5 mg/dL. Further lab workups were negative for hepatitis B virus (HBV), hepatitis C virus (HCV), human immunodeficiency virus (HIV), cytomegalovirus (CMV), Epstein-Barr virus (EBV), Strongyloides, C diff and fungal beta-D-glucan.
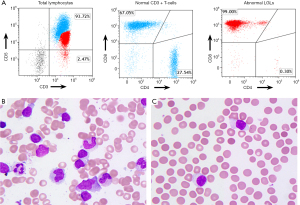
Bone marrow biopsy performed at our institute showed large lymphocyte aggregates, making up to 20% of total cellularity, with absent erythroid progenitor cells. Bone marrow flow cytometry demonstrated a distinct abnormal population of CD8+/CD5-dim CD3-positive T cells (Figure 1A), which also expressed CD2, CD45, CD16 and CD57. Frequent LGL cells were identified in bone marrow aspirate smear and peripheral blood smear (Figure 1B,1C). Polymerase chain reaction (PCR) of T-cell receptor gamma gene confirmed the clonality. A primary immunodeficiency gene panel was performed outside, which analyzed 429 genes that are associated with inherited disorders of the immune system (Invitae, San Francisco, CA, USA). STAT3 and STAT5b mutations were not identified, however a germline heterozygous missense mutation in BACH2 (c.31A>C; p.Met11Leu) was found.
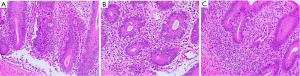
Colonoscopy showed colon and rectum was diffusely edematous with boggy and scalloped mucosa along with loss of vascular markings. No ulcers or erosions were seen. Segmental biopsies were obtained from the right colon to rectum. Microscopically, these colorectal biopsies showed intraepithelial lymphocytosis, prominent “popcorn” like crypt apoptotic bodies, scattered crypt abscess and absence of Paneth cells and goblet cells in the entire colon. Only focal mild crypt distortion was noted in the rectum (Figure 2A-2C). These histologic findings were most compatible with autoimmune enteropathy, which was further confirmed by positive serology for an anti-enterocyte antibody. Patient took budesonide 9 mg daily and oral magnesium, potassium and calcium. Patient’s symptoms became stable to better after a month. For the T-LGL, the patient was treated with prednisone and cyclosporine.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki and its subsequent amendments. Publication of this case report and accompanying images was waived from patient consent according to the Ethics Committee of University of Rochester Medical Center.
Discussion
T-LGL has been known as an indolent disease since initially described in 1985. Disease-related deaths are mainly caused by infections related to associated autoimmune disorders and immunosuppressive therapy. Autoimmune disorders can involve many different organ systems, such as endocrine, skeletal, muscular, circulatory, vascular, renal and digestive systems. Our patient presented with type I diabetes mellitus, pure red cell aplasia and celiac disease, as well as autoimmune enteropathy, which is a rarely reported association compared to other autoimmune diseases.
The patient had been on a gluten free diet for her celiac disease. In addition, her symptoms were not worsened by taking a gluten diet. Therefore, chronic diarrhea caused by celiac disease is unlikely for this patient. Patient’s pan-colonic autoimmune enteropathy is most likely the underlying cause for the chronic diarrhea. The duodenum is the most commonly affected location of autoimmune enteropathy; however, involvement of the colon and stomach has also been reported (9). This disease is manifested by the entire loss of Paneth and goblet cells, brisk apoptotic activity and intraepithelial lymphocytosis. Focal crypt abscess and distortion identified in rectum can mimic IBD histologically. Of note T-LGL has been reported to be associated with ulcerative colitis (5). Paneth cell metaplasia in the distal colon is an important manifestation of chronicity in IBD. The findings of only focal and mild crypt chronic changes and diffuse absence of Paneth and goblet cells help to differentiate it from IBD. Positive antibody serology test to enterocyte further supports a diagnosis of autoimmune enteropathy.
Autoimmune enteropathy can occur in the context of IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked) syndrome, which is caused by loss-of-function mutations in the FOXP3 gene on the X chromosome, however, no such mutations have been identified in this patient. Somatic activating STAT3 mutations have been identified in 40% to 70% of T-LGL patients, which become important features in the diagnosis of T-LGL (10,11). Of note, activating STAT3 mutations have also been reported to be involved in early-onset of multiple organ autoimmune diseases. STAT3 mutations were found in about 16% of patients with autoimmune enteropathy (3). STAT3 and STAT5b mutations were not found in our patient, however, a germline missense mutation in BACH2 gene was identified.
Bach2 is a member of the BTB-basic leucine zipper domain (bZIP) superfamily of transcriptional regulators. Bach2 can either form dimers with other transcription factors and translocate into the nucleus or directly interact with target DNA loci through Maf recognition elements (MARE), thereby inhibiting gene activation. It participates in oxidative stress-induced apoptosis through repression of the antiapoptotic factor HMOX1. It acts as a repressive “guardian” regulating adaptive immunity, maintaining regulatory T-cell function and B-cell maturation. Deficiency of BACH2 is associated with various autoimmune diseases, including asthma, insulin-dependent diabetes mellitus, Crohn’s disease, celiac disease and multiple sclerosis (12-15). BACH2 deficiency-related immunodeficiency is characterized by decreased FOXP3 expression in Treg cells and increased expression of T-bet and gut homing receptors, CCR9 and β7-integrin on CD4+ T cells (12,13). It still remains unclear whether the presence of the BACH2 mutation is involved in the pathophysiologies of this patient’s autoimmune diseases and T-LGL.
Conclusions
To our knowledge, this is the first reported case of autoimmune enteropathy associated with T-LGL with a co-existence of BACH2 mutation. Further investigation of its functional changes and pathogenesis in further studies in patients with T-LGL and concurrent autoimmune disorders is warranted.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-804/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-804/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-804/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki and its subsequent amendments. Publication of this case report and accompanying images was waived from patient consent according to the Ethics Committee of University of Rochester Medical Center.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lamy T, Moignet A, Loughran TP Jr. LGL leukemia: from pathogenesis to treatment. Blood 2017;129:1082-94. [Crossref] [PubMed]
- Marchand T, Lamy T, Loughran TP Jr. A modern view of LGL leukemia. Blood 2024;144:1910-23. [Crossref] [PubMed]
- Flanagan SE, Haapaniemi E, Russell MA, et al. Activating germline mutations in STAT3 cause early-onset multi-organ autoimmune disease. Nat Genet 2014;46:812-4. [Crossref] [PubMed]
- Haapaniemi EM, Kaustio M, Rajala HL, et al. Autoimmunity, hypogammaglobulinemia, lymphoproliferation, and mycobacterial disease in patients with activating mutations in STAT3. Blood 2015;125:639-48. [Crossref] [PubMed]
- Kondo H, Watanabe J, Iwasaki H. T-large granular lymphocyte leukemia accompanied by an increase of natural killer cells (CD3-) and associated with ulcerative colitis and autoimmune hepatitis. Leuk Lymphoma 2001;41:207-12. [Crossref] [PubMed]
- Kondoh K, Morimoto M, Keino D, et al. T-cell large granular lymphocyte leukemia in a child with anemia and Crohn's disease. Pediatr Int 2013;55:111-4. [Crossref] [PubMed]
- Fouquet G, Rossignol J, Ricard L, et al. BLNK mutation associated with T-cell LGL leukemia and autoimmune diseases: Case report in hematology. Front Med (Lausanne) 2022;9:997161. [Crossref] [PubMed]
- Ullah F, Markouli M, Orland M, et al. Large Granular Lymphocytic Leukemia: Clinical Features, Molecular Pathogenesis, Diagnosis and Treatment. Cancers (Basel) 2024;16:1307. [Crossref] [PubMed]
- van Wanrooij RLJ, Bontkes HJ, Neefjes-Borst EA, et al. Immune-mediated enteropathies: From bench to bedside. J Autoimmun 2021;118:102609. [Crossref] [PubMed]
- Koskela HL, Eldfors S, Ellonen P, et al. Somatic STAT3 mutations in large granular lymphocytic leukemia. N Engl J Med 2012;366:1905-13. [Crossref] [PubMed]
- Fasan A, Kern W, Grossmann V, et al. STAT3 mutations are highly specific for large granular lymphocytic leukemia. Leukemia 2013;27:1598-600. [Crossref] [PubMed]
- Afzali B, Grönholm J, Vandrovcova J, et al. BACH2 immunodeficiency illustrates an association between super-enhancers and haploinsufficiency. Nat Immunol 2017;18:813-23. [Crossref] [PubMed]
- Trujillo-Ochoa JL, Kazemian M, Afzali B. The role of transcription factors in shaping regulatory T cell identity. Nat Rev Immunol 2023;23:842-56. [Crossref] [PubMed]
- Mazzieri A, Montanucci P, Basta G, et al. The role behind the scenes of Tregs and Th17s in Hashimoto's thyroiditis: Toward a pivotal role of FOXP3 and BACH2. Front Immunol 2022;13:1098243. [Crossref] [PubMed]
- Hu Q, Xu T, Zhang M, et al. Diverging regulation of Bach2 protein and RNA expression determine cell fate in early B cell response. Cell Rep 2022;40:111035. [Crossref] [PubMed]


