
Multi-omics analyses develop and validate the optimal prognostic model on overall survival prediction for resectable hepatocellular carcinoma
Highlight box
Key findings
• This study found that the mutations of FBN1 and MAP1B, the transcription of CCNJL, FRMD1, GRPEL2, and the aberrant methylation of CXorf15, DACT2, GP6, KIAA1522, and PDIA3 were independent risk factors for the overall survival (OS) of resectable hepatocellular carcinoma (HCC). A multi-omics prognostic model for OS was established. Internal and external validation achieved an optimal performance.
What is known and what is new?
• Previous studies have investigated the alterations of HCC at genetic, transcriptional, or epigenetic levels, but rarely combined multi-omics for HCC prognosis prediction.
• A multi-omics model combining molecular aberrancies and clinicopathological information was established and proved to be optimal for prognosis prediction of resectable HCC.
What is the implication, and what should change now?
• The multi-omics model can provide risk factor information, better stratification, and optimization of treatment strategies, which is helpful for therapeutic strategy selection and survival assessment.
Introduction
Hepatocellular carcinoma (HCC) is the sixth leading cause of morbidity and the third leading cause of mortality (1) in all cancers in the world. In Europe and the United States, HCC mainly develops from liver cirrhosis caused by alcoholic liver disease, fatty liver disease, and hepatitis C virus (HCV) infection. In East and Southeast Asia, HCC mainly develops from liver cirrhosis caused by hepatitis B. In recent years, the ratio of HCC caused by alcoholic liver cirrhosis is rising fast (2). The treatment strategies of HCC are definite at present based on the staging. For early and middle stage HCC, the main treatment methods include surgical resection, ablation, and transcatheter arterial chemoembolization (TACE) (3). For advanced HCC, systematic therapy based on immunotherapy and multi-target inhibitors is the primary approach (3). The prognosis of HCC is satisfactory if early HCC can be cured, and the 5-year survival rate can exceed 80%, while the prognosis of advanced HCC is poor, and the 5-year survival rate is less than 20% (4). Therefore, early detection and early treatment is the main strategy to improve the cure rate and survival rate of HCC.
Many studies have been conducted to investigate the pathogenesis of HCC in terms of genomic, transcriptomic, and epigenomic alterations, and some of them focused on the relationship between alterations and therapeutic response or prognosis. In genomics, mutations of TP53, TERT promoter, CTNNB1, AXIN1, ARID1A, and other genes have been found to be high-frequency mutations in HCC (5). Amplifications of CCND1, FGF19, MYC, MET, VEGFA, MCL1, and TERT, as well as RB1, CDKN2A, ERRFI1, NCOR1, and PTEN deletions are the common copy number changes (5). Some of these amplifications are found to be associated with poor immunotherapy response and hyperprogressive disease (6,7).
Previous studies on HCC transcriptome identified a huge amount of differentially upregulated and downregulated genes (8-10), and some of these genes can be related to clinical characteristics (9) or prognosis (11,12). For example, one study identified a signature composed of 11 disulfidptosis and ferroptosis (DRG-FRG)-related genes as a promising prognostic biomarker for HCC, which improved our understanding on the crosstalk between DRG-FRG genes related to HCC immunotherapy (13). Another study developed a chromatin regulator (CR)-based signature and demonstrated its predictive capabilities for HCC prognosis, independence from other clinical factors (14). Similarly, a study developed a prediction model for HCC prognosis based on the pathomics signatures (PS) and the genomics signatures (GS), and established a nomogram model which could effectively predict the survival probability of HCC patients (15). In contrast, one study demonstrated that the expression of a single gene, NDC1 might serve as a valuable predictor in the prognosis and response to immunotherapy of HCC (16).
In addition, current studies have found that methylation alterations are widespread in HCC (17,18). These methylation alterations were suggested to be closely related to HCC prognosis. For example, cell-free DNA (cfDNA) methylation was reported to be capable of monitoring patient response and predicting prognosis (19,20). These single-omics studies found that the main aberrant pathways of HCC include Ras/Raf/MAPK pathway, PI3K/AKT/mTOR pathway, Notch pathway, Wnt/β-catenin pathway, and TGF-β pathway, etc. (21).
Although the above reviewed studies have focused on single-omics aberrancies and achieved good efficacy, the research on prognostic prediction still needs to be conducted at multi-omics level, since heterogeneity across samples and populations and bias related with detection techniques may exist when investigating the prognosis based on single-omics. Furthermore, models based on multi-omics markers may provide multiple dimensional information to minimize the overfitting or underfitting during model construction. There is currently a lack of clinically recognized prognostic markers for HCC, especially for patients with early and middle stage HCC with resectable or ablable lesions (stage I–IIIA HCC). Meanwhile, prognostic studies require long-term follow-up, making it more difficult to identify reliable prognostic markers. To address these tissues, in this study, we made use of the existing standardized public database to systematically study the alterations of HCC at genomic, transcriptomic, and epigenomic levels, and identified several single-omics risk factors significantly associated with patient prognosis. By establishing single-omics prognosis prediction models, and conducted real sample verification, we confirmed the validity of these markers and models. Subsequently, we combined these significant risk factors from single-omics with clinicopathological information, and established a multi-omics prognosis prediction model, and conducted real sample validation. This was because clinicopathological information includes factors known to influence the prognosis, and inclusion of these factors may minimize the potential risk and bias of molecular markers when establishing a combined model, which could be more balanced and accurate with clinicopathological factors. Compared with single-omics models, the multi-omics model significantly improved the prognostic performance. The multi-omics model provided a powerful tool for risk factor analysis and prognosis prediction of patients with early and middle stage HCC, and may help patients to choose appropriate therapeutic strategies before treatment. We present this article in accordance with the TRIPOD reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-710/rc).
Methods
Ethics
This study was a database study combined with a retrospective cohort study. The study was designed and implemented at the Department of Hepatology and Gastroenterology, Beijing Youan Hospital, Capital Medical University. The study was approved by the Ethics Committee of Beijing Youan Hospital (approval No. [2018]015) and was implemented following the Declaration of Helsinki and its subsequent amendments. The waiver of informed consent was granted by the hospital Ethics Committee due to the retrospective use of clinical samples in this study. Patient information was strictly confidential, and the testers did not know the patient information.
Clinical study design, patient recruitment, and sample collection
Subjects were recruited following the inclusion criteria, including adult patients with complete clinicopathological and follow-up information, and a confirmed diagnosis of stage I–IIIA HCC. The criteria were identical for patients in The Cancer Genome Atlas (TCGA) database and patients from Beijing Youan Hospital’s sample bank. Patients were recruited for those who underwent lesion surgical resection, any type of ablation therapy and TACE, with subsequent chemotherapy or combined chemo- and radiotherapy. For patients involved in external validation cohort, the accessibility of primary cancer tissue samples and sample information was also a requirement. All included patients should have complete follow-up information, at least the overall survival (OS) information was required. Stage IIIB and IV HCC patients, patients with a previous history of malignancies, and patients whose samples or follow-up information were not available were excluded. Samples that failed to pass the quality controls in any study step including the next-generation sequencing (NGS) were also excluded. The follow-up information included the outcome data on patient death or survival and the specific length of time, which was used to assess the OS.
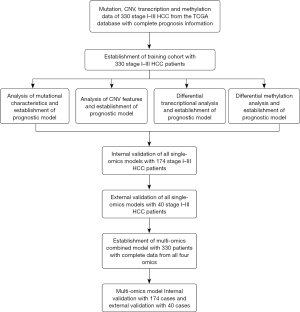
Data from the TCGA database (https://portal.gdc.cancer.gov/) were used for the training cohort. A total of 330 stage I–IIIA HCC patients were involved based on the above inclusion and exclusion criteria (Table 1). The estimation of sample size for model establishment was based on previous guidance (22). In brief, the c statistic was about 0.9 based on previous experience, and the event proportion was about 0.1. We planned to include five parameters in the models, and the required sample size was 4× events per parameter (EPP), equivalent to 20 events. Therefore, the total minimal sample size was 20/0.1=200 cases. All patients were involved in mutational, copy number variation (CNV), transcriptional, and methylation analyses and the establishment of single-omics models. The establishment of the multi-omics model used data and results of analysis from all four omics (Table 1, Figure 1). Internal validation cohort was established by randomly selecting 174 samples (roughly half of the total available samples, ratio at 2:1) from the 330 stage I–IIIA HCC patients. External validation was established by collecting 40 retrospective samples from the sample bank of Department of Hepatology and Gastroenterology, Beijing Youan Hospital’s previous research cohort and sending all samples for NGS sequencing (Table 1, Figure 1). The criteria for selecting samples for external validation was identical to that for the training cohort. Samples, the OS, clinicopathological, and prognosis information were collected. Table 1 shows a summary of patient clinicopathological information for all three cohorts, and the characteristics of patients are described in the first paragraph of the results section. The flowchart in Figure 1 shows the procedure of patient selection, data analysis, and model training and validation.
Table 1
| Factors | Training | Internal validation | External validation |
|---|---|---|---|
| Total | 330 (100.0) | 174 (100.0) | 40 (100.0) |
| Race | |||
| Asian | 168 (50.9) | 93 (53.4) | 26 (65.0) |
| American Indian or Alaska Native | 2 (0.6) | 0 (0.0) | 0 (0.0) |
| Black or African American | 8 (2.4) | 8 (4.6) | 1 (2.5) |
| White | 144 (43.7) | 69 (39.7) | 13 (32.5) |
| Not reported | 8 (2.4) | 4 (2.3) | 0 (0.0) |
| Gender (male) | 216 (65.5) | 128 (73.6) | 21 (70.0) |
| Stage | |||
| Stage I | 192 (58.2) | 83 (47.7) | 14 (46.7) |
| Stage II | 77 (23.3) | 53 (30.5) | 9 (30.0) |
| Stage III | 61 (18.5) | 38 (21.8) | 7 (23.3) |
| Age (years) | 59.62 (6.33) | 59.92 (14.03) | 56.43 (16.58) |
| TMB (mut/Mb) | 1.74 (0.75) | 2.04 (2.79) | 2.09 (3.05) |
| Residual tumor | |||
| R0 | 298 (90.3) | 153 (87.9) | 28 (93.3) |
| R1 | 14 (4.2) | 14 (8.1) | 2 (6.7) |
| RX | 18 (5.5) | 7 (4.0) | 0 (0.0) |
| Vascular tumor cell type | |||
| Micro | 184 (55.8) | 105 (60.3) | 27 (67.5) |
| Macro | 26 (7.9) | 21 (12.1) | 13 (32.5) |
| Not reported | 120 (36.3) | 48 (27.6) | 0 (0.0) |
| Child-Pugh classification grade | |||
| A | 208 (63.0) | 111 (63.8) | 33 (82.5) |
| B | 10 (3.0) | 13 (7.5) | 7 (17.5) |
| C | 0 (0.0) | 1 (0.6) | 0 (0.0) |
| Not reported | 112 (34.0) | 49 (28.1) | 0 (0.0) |
Data are presented as n (%) or mean (SD). HCC, hepatocellular carcinoma; SD, standard deviation; TMB, tumor mutational burden.
Whole-exome sequencing (WES) and data processing
WES was used for mutation and CNV detection. All experiments were performed on the Illumina Novaseq 6000 platform as previously described (23,24). Data were generated and processed as previously described (23,24).
RNA sequencing (RNA-seq) and data processing
RNA-seq was used for RNA level measurement. RNA was extracted from cancer and adjacent normal tissues using TruSeq Targeted RNA Expression Library Prep Kit (Illumina, Shanghai, China). Data were generated and processed as previously described (7,25).
Whole-genome bisulfite sequencing (WGBS) and data processing
WGBS was used for methylation level measurement. DNA was extracted from cancer tissue and adjacent normal tissues using the QIAamp DNA FFPE tissue kit (Qiagen, Shanghai, China). Data were generated and processed as previously described (26,27).
Data and statistical analysis
Mutation and CNV data were analyzed using the “maftools” package of the R software (https://www.r-project.org/). The ‘Complex Heatmaps’ R package was used to generate and plot the mutational landscape (23,24). The “DESeq2” R package was used to analyzed the messenger RNA (mRNA) transcriptional data, and the volcano plot and heatmap were generated from the differential transcription data (7,25). The transcription clustering was generated based on the calculation of the transcripts per kilobase of exon model per million mapped reads (TPM) values. Patients were dichotomized based on the TPM values. The differential methylation analysis was performed with the R software “ChAMP” package (26,27). Beta values of methylation data were calculated by Euclidean distance, and patients were divided into high and low methylation groups. The threshold for log2fold change was set at 3-fold standard deviations of the mean values, and the threshold for P values was set at 0.05 [−log10(0.05)=1.3].
The “clusterProfiler” R package was used for enrichment analyses. The “survival” and “survminer” packages were used for the Kaplan-Meier survival analysis and log-rank (Mantel-Cox) test. The receiver operating characteristic (ROC) curves were plotted and the area under the curve (AUC) was calculated using the “survivalROC” R package. Univariate Cox regression analysis was performed for single-omics and multi-omics data. The least absolute shrinkage and selection operator (LASSO) regression was performed for variable selection by using the “glmnet” R package. Multivariate Cox analyses were performed with significant or close-to-significant variables from univariate analyses. Significant candidate genes from multivariate analyses were used for the establishment of nomogram scheme. The optimal model was identified by examining the significance of various parameter combinations using the Wald test and likelihood ratio test. The selection of parameters for the final multi-omics model was based on the selection for optimal AUC. The minimal acceptable AUC for internal validation and external validation was 0.70 and 0.65, respectively, for single-omics models, and was 0.80 and 0.75, respectively, for the multi-omics model. Benjamini & Hochberg (BH) method was used for P value correction. P<0.05 (two-sided) was considered statistically significant.
Results
Characterization of mutational alterations in HCC and establishment of a prognostic model
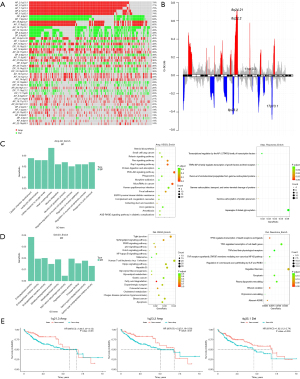
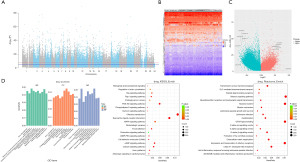
The demographic and clinicopathological information of patient involved in training, internal validation, and external validation cohorts is summarized in Table 1. Asian and white are the two main races in all cohorts, and male was the predominant sex. Approximately half of the patients were at stage I in all cohorts, followed by stage II and III. Over 90% of patients achieved R0 resection, and more than half of the patients showed microvascular invasion while the rest showed macrovascular invasion. Most patients achieved Child-Pugh classification grade A. To investigate the prognostic factors in each omics, the mutational, copy number, transcriptional, and methylation profiles in HCC were characterized first. Figure 2 shows the mutational characteristics and results of enrichment analysis in HCC. Figure 2A shows the mutational landscape of 330 HCC patients involved in this study. TP53, CTNNB1, and TTN were among the genes with the top mutational frequency, while CSMD1, TP53, and RB1 were among those with the most CNV alterations. The mutational characteristics are summarized in Figure 2B, in which single-nucleotide polymorphism (SNP) was the dominant variant type and missense mutations were the main mutation type. TP53, TTN, and CTNNB1 were genes with the most mutations. Figure 2C shows the landscape of co-mutations and exclusive mutations. There were quite a few co-mutations, such as DNAH7/CACNA1E and APOB/CTNNB1. In contrast, there were only two pairs of significant exclusive mutations, i.e., AXIN1/DOCK2 and AXIN1/CTNNB1. The enrichment analysis on mutations is shown in Figure 2D. Gene Ontology (GO) enrichment showed aberrancies in cell skeleton, cell adhesion, cell junction, cell development, and Kyoto Encyclopedia of Genes and Genomes (KEGG) and Reactome enrichments showed abnormalities in a series of key functions or pathways, such as protein digestion and absorption, calcium signaling pathway and extracellular matrix organization.

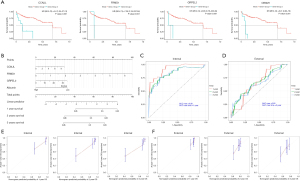
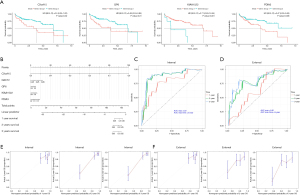
Univariate analysis was performed to identify risk factors for prognosis. Table available at https://cdn.amegroups.cn/static/public/jgo-24-710-1.xlsx shows that FBN1 and MAP1B mutations were significant factors in univariate analysis, while mutations of several genes significantly stratified the patient OS (Figure 3A). Subsequent multivariate analysis in Table 2 identified FBN1 and MAP1B mutations, stage, residue tumor, and vascular tumor cell type as independent risk factors for prognosis, which were used to establish a Cox model for prognosis prediction (Figure 3B). Internal and external validations were performed and found that the optimal performance was achieved at 1 year with an AUC of 0.77 for internal validation (Figure 3C), and at 3 years with an AUC of 0.68 for external validation (Figure 3D). Linear regression analyses between actual OS and nomogram-predicted probability of OS at 1, 2, and 3 years also proved the effectiveness of the model in both internal validation (Figure 3E) and external validation (Figure 3F).

Table 2
| Factors | Univariant variables | Multiple variables | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| FBN1 | |||||
| Mut | Reference | Reference | |||
| WT | 0.183 (0.07–0.475) | <0.001* | 0.12 (0.04–0.36) | <0.001* | |
| MAP1B | |||||
| Mut | Reference | Reference | |||
| WT | 0.154 (0.054–0.435) | <0.001* | 0.1206 (0.040–0.360) | <0.001* | |
| Stage | |||||
| I | Reference | Reference | |||
| II | 1.100 (0.516–2.344) | 0.81 | 2.47 (1.17–5.20) | 0.21 | |
| III | 2.292 (1.168–4.497) | 0.02* | 0.00 (0.00–Inf) | 0.02* | |
| Residual tumor | |||||
| R0 | Reference | Reference | |||
| R1 | 1.515 (0.360–6.380) | 0.57 | 0.68 (0.11–4.24) | 0.68 | |
| R2 | NA (NA–NA) | NA | NA (NA–NA) | NA | |
| RX | 3.844 (1.328–11.125) | 0.01* | 5.31 (1.75–16.17) | 0.003* | |
| None | 0.000 (0.000–Inf) | >0.99 | 0.68 (0.11–4.24) | >0.99 | |
| Vascular tumor cell type | |||||
| Micro | Reference | Reference | |||
| Macro | 3.656 (0.984–13.585) | 0.05* | 1.71 (0.74–3.96) | 0.03* | |
| NA | 1.237 (0.589–2.596) | 0.57 | 0.00 (0.00–0.00) | 0.21 | |
*, P≤0.05. CI, confidence interval; HR, hazard ratio; Inf, infinite; Mut, mutation; NA, not applicable; RX, residual condition unknown; WT, wild type.
Characterization of CNVs in HCC
The CNVs were then characterized. The CNV landscape in Figure 4A shows the top CNVs in HCC, in which many amplifications and deletions can be found. 1q21.3 and 1q23.3 ranked the highest in amplifications and 8p12 and 8p23.3 ranked the highest in deletions. Figure 4B shows the distribution of the most prominent amplifications and deletions, including amplifications of 8q24.21, 8q22.2, 11q13.3 and deletions of 8p23.3 and 17p13.1. Enrichment analysis on amplifications in Figure 4C shows that the aberrant pathways or functions mainly included cell apoptotic process, PI3K-Akt pathway and microRNAs. Enrichment analysis on deletions in Figure 4D shows that the aberrant pathways or functions mainly included peptidase activity, fatty acid degradation and TP53 signaling. Univariate analysis of CNVs on prognosis did not find any significant factors (table available at https://cdn.amegroups.cn/static/public/jgo-24-710-2.xlsx), and therefore no model can be established for prognosis prediction. However, three CNVs significantly stratified the patient survival, including amplifications of 1q21.3, 1q23.3 and deletions of 4q35.1 (Figure 4E).
Characterization of transcriptional alterations in HCC and establishment of a prognostic model
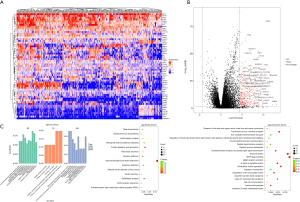
The characterization of transcription identified many potential aberrantly transcripted genes. The heatmap in Figure 5A shows some representative aberrantly transcripted genes, including AFP, GPC3, TERT, and CDH9. Both upregulation and downregulation can be found, while the number of significant upregulated genes far overweight that of the down-regulated genes, which can be clearly observed in the volcano plot in Figure 5B. CDH9 and TERT were among those with the most prominent upregulation. GO, KEGG, and Reactome enrichment analyses in Figure 5C shows that the aberrant functions or pathways mainly included organ development, receptor and synapse function/activity, neuroactive ligand-receptor interaction, and G protein-coupled receptor (GPCR) ligand binding.
Univariate analysis identified 2,516 significant genes out of 19,431 genes (table available at https://cdn.amegroups.cn/static/public/jgo-24-710-3.xlsx), and subsequent multivariate analysis identified CCNJL, FRMD1, GRPEL2, and albumin as independent risk factors (Table 3). The representative gene for stratification of patient survival is shown in Figure 6A. These significant factors were used in establishing a prognostic model, which is shown in Figure 6B. Internal validation and external validation achieved the maximal AUC of 0.93 at 1 year and 0.65 at 2 years, respectively (Figure 6C,6D). Linear regression between actual OS and nomogram-predicted probability of OS at 1, 2, and 3 years also proved the effectiveness of the model in both internal validation (Figure 6E) and external validation (Figure 6F).
Table 3
| Factors | Univariant variables | Multiple variables | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| CCNJL | 1.505 (1.314–1.724) | <0.001* | 1.45 (1.18–1.78) | <0.001* | |
| FRMD1 | 1.621 (1.286–2.042) | <0.001* | 4.74 (2.44–9.20) | <0.001* | |
| GRPEL2 | 1.161 (1.11–1.214) | <0.001* | 1.09 (1.02–1.18) | 0.02* | |
| Albumin | |||||
| Normal | Reference | Reference | |||
| High | 1.539 (0.207–11.441) | 0.67 | 0.00 (0.00–0.08) | 0.001* | |
| Low | 1.175 (0.528–2.617) | 0.69 | 1.68 (0.72–3.96) | 0.23 | |
*, P≤0.05. CI, confidence interval; HR, hazard ratio.
Characterization of methylation alterations in HCC and establishment of a prognostic model
The alterations in methylation were also investigated. The Manhattan plot in Figure 7A shows the distribution of methylation status across all chromosomes, and many aberrant methylation sites can be observed. The profile of upregulation and downregulation is presented as the heatmap in Figure 7B, and the significant methylation sites are presented as volcano plot in Figure 7C, in which both hypermethylation and hypomethylation can be observed. GO, KEGG, and Reactome enrichment analyses in Figure 7D found that the aberrant functions or pathways mainly included synaptic function, receptor and ion channel activity, olfactory transduction and signaling pathway, neuroactive ligand-receptor interaction, and GPCR ligand binding.
Univariate analysis identified five significantly methylated genes out of 15,842 genes (table available at https://cdn.amegroups.cn/static/public/jgo-24-710-4.xlsx), and subsequent multivariate analysis identified CXorf15, DACT2, GP6, KIAA1522, and PDIA3 as independent risk factors (Table 4). Stratification of survival by methylation status of representative genes is shown in Figure 8A. These significant factors were used to establish a prognostic model, shown in Figure 8B. Internal and external validations found maximal AUC of 0.9 at 3 years and 0.81 at 3 years, respectively (Figure 8C,8D). Linear regression between actual OS and nomogram-predicted probability of OS at 1, 2, and 3 years also proved the effectiveness of the model in both internal validation (Figure 8E) and external validation (Figure 8F).
Table 4
| Factors | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| CXorf15 | 0 (0–0.014) | 0.04* | 0.00 (0.00–0.01) | 0.04* | |
| DACT2 | 0.007 (0–0.914) | 0.04* | 0.00 (0.00–0.07) | 0.008* | |
| GP6 | 0.073 (0.016–0.342) | 0.001* | 0.08 (0.01–0.65) | 0.03* | |
| KIAA1522 | 0.007 (0–0.087) | <0.001* | 0.00 (0.00–0.05) | <0.001* | |
| PDIA3 | 323,381.111 (56.688–1,844,759,081.421) | 0.004* | 751,275.71 (17.14–32,935,962,312.03) | 0.01* | |
*, P≤0.05. CI, confidence interval; HR, hazard ratio.
Establishment and optimization of a multi-omics prognostic model
The significant factors from mutational, CNV, transcriptional and methylation analysis, combined with clinicopathological factors, were used to establish a prognostic multi-omics model. Figure 9A shows that the optimized model was composed of cancer stage, methylation of six genes, and transcription of eight genes. The model can be used to predict the patient survival by calculating the score from each factor. Internal validation in Figure 9B shows an optimal maximal AUC of 0.98 at 1 year. Subsequent external validation in Figure 9C shows an optimal maximal AUC of 0.88 at 2 years. Linear regression between actual OS and nomogram-predicted probability of OS at 1, 2, and 3 years also proved the effectiveness of the model in both internal validation (Figure 9D) and external validation (Figure 9E).

Discussion
The objectives of this study were to establish a prognostic model combining multiple omics and clinical information to predict the prognosis of patients with surgically resectable HCC, and to assist patients in selecting appropriate treatment strategies before surgery. In this study, by downloading and analyzing data from existing standardized database on mutations, CNVs, transcriptome and methylation, as well as corresponding clinical information, we first trained single-omics prognostic prediction models, identified independent risk factors that were significantly associated with prognosis for each single-omics, and conducted internal and external validation of the models. Due to the limited predictive performance of single omics, we combined the independent risk factors identified from each single omics with clinicopathological factors to establish a multi-omics prognostic prediction model, and conducted internal and external validation of the model through database and testing retrospective samples to optimize the model performance, and finally achieved the optimal performance. In this study, we not only defined the main alteration characteristics, functions and pathways of HCC in terms of mutation, CNV, transcription, and methylation, but also identified significant risk factors, and established single-omics and multi-omics models. The multi-omics model may help patients choose treatment strategies before treatment.
Previous studies have investigated the landscape of HCC mutations and their relationship to prognosis. The most frequent CNVs in HCC were found to be amplifications in chromosome 6p21 (VEGFA) and 11q13 (FGF19/CNND1) and homozygous deletions in chromosome 9 (CDKN2A), while the most frequent mutations were found with TERT promoter (60%), TP53 and CTNNB1 (25–30%), in addition to low-frequency mutated genes (e.g., AXIN1, ARID2, ARID1A, TSC1/TSC2, etc.) (28). Genomic profiling data suggested two major molecular clusters (proliferation and nonproliferation) of HCC, with differential enrichment in prognostic signatures, pathway activation, and tumor phenotype (28). A study published in 2017 systematically reported the mutational landscape of HCC (5). In addition to the above identified mutations, the authors made a detailed analysis on TERT mutations. TERT promoter mutations were found to correlate with higher age (P=0.0006), male (P=0.006), HCV positive (P=0.04), and non-hepatitis B virus (HBV) positive (P=0.02) (5). TERT promoter mutation also strongly correlated with CDKN2A silencing by promoter hypermethylation. The CDKN2A gene encodes the tumor suppressor p16INK4A. The p16INK4A expression downregulation was in conjunction with enhanced TERT expression, which may be essential for epithelial cell immortalization, a cancer hallmark (29). Therefore, the population characteristics of TERT mutations and a series of epigenetic and transcriptional changes may have a significant impact on patient prognosis. The tumor mutation burden (TMB) is a stratification marker that has an important impact on the response and prognosis of patients in the era of immunotherapy. One study found that TMB values were associated with the patient risk, and the higher-risk group had a higher proportion of TP53 mutations than the low-risk group (30). The significance of co-mutations and mutually exclusive mutations has rarely been study in HCC, while these mutational characteristics may reflect the alterations in aberrant pathways and may potentially be the specific molecular markers for HCC, especially when differential diagnosis is needed.
CNV is a prominent feature of tumors and is widely found not only in cancer, but also in the pre-cancer tissues. Clinical evidence has found that copy number changes in HCC are significantly associated with response and prognosis. One study performed Cox regression analysis and indicated that the CNVs were significantly associated with OS in 192 genomic regions (P<0.05). In the analysis of tumor recurrence, 514 genomic regions with CNVs were associated with recurrence. Integrative analysis revealed that the overexpression of 16 genes was significantly associated with shorter time to tumor recurrence. Furthermore, the low expression of TLE4 and XPA was associated with poor OS. In multivariate analysis, FGR and XPA were independent risk factors of early recurrence and poor OS, respectively (31). Another study reported that SF3B4 expression was significantly higher in cancerous than in non-cancerous tissues and positively correlated with SF3B4 copy number. High SF3B4 expression was significantly associated with intrahepatic metastasis and poor prognosis (32). Recent studies have found that changes in copy number are associated with immunotherapy efficacy. For example, MDM2/4 and FGF/3/4/19 amplifications were suggested to correlate with hyperprogressive disease or high ratio of tumor progression in HCC (6,7). Chromosome 11q13 amplification was also reported to correlate with poor response and prognosis to PD-1 blockade in unresectable HCC (33). The amplifications with significant stratification on OS (1q21.3, 1q23.3, and 4q35.1) have not been reported in previous studies, and their roles are worth further investigation.
The relationship between transcriptional alterations and prognosis has also attracted much attention in HCC. The transcription of various signaling pathways including telomere maintenance, Wnt/β-catenin, P53/cell cycle regulation, oxidative stress, AKT/mTOR, and MAP kinase is frequently altered in HCC, and several HCC subtypes have been identified based on transcriptomic dysregulation, which may be related to risk factors, pathological features and prognosis (34). Prognosis or survival-related risk models based on transcriptional alterations were established by quite a few studies. For example, one study revealed a 14-gene signature extracted from differentially expressed gene (DEG) hub genes and found that it not only served as a predictive signature for HCC outcome but could also be used to predict HCC recurrence (35). Another study developed nuclear mitochondrial-related genes (NMRGs) signature, which helped to predict prognosis and tumor microenvironment, and provided potential targeted therapies for HCC patients with different NMRG prognosis scores (11). A more recent study used single-cell RNA sequencing (scRNA-seq) data and developed a 3-gene signature (CLTA, TALDO1, and CSTB) based on the heterogeneity of the tumor immune microenvironment to predict the survival outcome and immunotherapy response (36). A study also combined TP53 mutation status and RNA expression in different populations and platforms and developed an immune prognostic model (IPM) based on immune-related genes that were differentially expressed between wild-type TP53 and mutant TP53 HCC samples. The authors suggested that IPM, which was sensitive to TP53 mutation status, may have important implications for identifying subgroups of HCC patients with low or high risk of unfavorable survival (37). Since transcriptome is substantially altered in HCC, it can be seen from the above studies that the detection of transcriptional alterations may be a powerful tool to develop models for prognosis prediction. However, due to the inter- and intra-tumoral heterogeneity of transcriptional status among different subtypes, studies are still needed to identify stable markers related to prognosis. The transcription levels of proteins including AFP, GPC3, and TERT were found to be upregulated in this study. These proteins have been widely reported in previous studies and used as markers for HCC diagnosis (38). In contrast, independent risk factors including CCNJL, FRMD1, GRPEL2, and OR6M1 have rarely been reported, and could potentially become new markers for HCC prognosis.
The alterations of methylation in HCC are comprehensive and prominent, and many studies have reported the applications of methylation markers in response assessment and prognosis prediction. One early study constructed a diagnostic prediction model using cfDNA samples from a large cohort of 1,098 HCC patients and 835 normal controls. The model showed high diagnostic specificity and sensitivity and was highly correlated with tumor burden, treatment response, and stage. The study also constructed a prognostic prediction model that effectively predicted prognosis and survival (19). Another study also found that the levels of m6A methylation correlated with HCC patient survival and prognosis, which implied that m6A methylation-related genes may be promising prognostic indicators or therapeutic targets for HCC (39). A more recent study also determined the m6A/m5C/m1A regulated genes in HCC tissues and constructed a risk model and nomogram. It was found that the cluster subgroup and risk model of m6A/m5C/m1A regulated genes were associated with poor prognosis and immune microenvironment and were potentially the new tools for assessing the prognosis of HCC patients (40). In addition, circulating tumor DNA (ctDNA) involving mutational, CNV, and methylation aberrancies have been proved to be emerging liquid biopsy biomarkers for therapeutic monitoring in HCC, which may greatly facilitate patient management and drug development (20). The independent risk factors identified in this study, such as CXorf15, GP6, KIAA1522, and PDIA3, appeared to be more effective than mutational and transcriptional factors in predicting OS when combined in a model. Therefore, the potential of methylation markers for prognosis prediction is worth further in-depth study.
In clinical practice, the model based on multi-omics can be used to predict the prognosis of patients before surgery, and the prediction efficiency is satisfactory. In the future, it may be possible to obtain the tumor tissue of patients before or after surgery, collect the mutation, CNV, transcriptome, and methylation information of the tumor tissue, and use the detection results to predict the survival of patients using the model established in this study. Meanwhile, it should be noted that blood-based tests and corresponding models may greatly facilitate the application of models in practice. If a good prognosis is expected, treatment according to the current standard strategy may be considered based on the stage of the patient’s cancer, and the patient’s response and recurrence may be closely monitored. If a bad prognosis is expected, it may be necessary to evaluate the current standard treatment strategy to shorten the review cycle and apply more aggressive adjuvant therapy, such as additional targeted therapy or immunotherapy or longer drug duration, in order to better postpone disease progression and reduce recurrence.
In recent years, ctDNA, as a non-invasive biomarker, has attracted much attention in the field of response monitoring and prognosis prediction of liver cancer. Many high-quality studies have revealed its great potential in the management of liver cancer (19,41). ctDNA can accurately reflect the genetic variation of liver cancer patients. Its mutational and methylation characteristics are not only closely related to the occurrence and development of liver cancer, but also can effectively evaluate the therapeutic effect and predict the recurrence of disease. For example, ctDNA mutations of TERT, TP53, and other genes occur frequently in patients with liver cancer, and are significantly correlated with tumor stage and poor prognosis (42). In addition, dynamic monitoring of ctDNA provides strong support for individualized therapy. Studies have shown that changes in ctDNA levels before and after treatment can reflect changes in tumor load in real time, which helps doctors to timely adjust the treatment plan and improve the survival rate of patients (19,41,42). Therefore, ctDNA, as a new biomarker, shows a broad prospect in monitoring the efficacy and predicting the prognosis of HCC. Future studies need to further explore its mechanism and optimize detection methods to better serve the clinical management of HCC.
There are some limitations in this study. Firstly, the cohort for external validation was relatively small, and the prognostic information was retrospective. In subsequent studies, the sample size can be expanded and prospective validation can be performed. Secondly, patients can be stratified in more detail according to treatment methods in the future, including surgical resection, ablation, TACE, etc., and prognosis and survival models can be established according to treatment methods. However, this requires sufficient sample size for each treatment method. Similarly, it is also necessary to consider the impact of postoperative adjuvant therapy on the prognosis and survival of patients and conduct detailed stratification. Thirdly, the recurrent-free survival (RFS) is crucial for the prognosis of resectable HCC patients, but it was not available in TCGA database, and future study may focus on the prediction of RFS by models. Fourthly, establishment of blood-based tests and corresponding models for prognosis prediction has advantages compared with tissue-based tests (19). Future studies should focus on blood ctDNA tests for prognostic models. Finally, there are still several transcription and methylation parameters included in this model, which can be further optimized in the future. Fewer parameters can be tested by polymerase chain reaction (PCR) instead of NGS to reduce testing costs and patient burden.
Conclusions
In this study, the mutational, copy number, transcriptional and methylation alterations in HCC were combined with clinicopathological information to establish and validate a multi-omics model for predicting the prognosis of patients with resectable HCC. The multi-omics prognostic prediction model can provide molecular level risk factor information, better stratification and management, and optimization of treatment strategies. This model may be helpful for therapeutic strategy selection and survival assessment.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-710/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-710/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-710/prf
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-710/coif). Y.H. reports that this study was supported by the Beijing Municipal Science and Technology Commission Project “Study and application of ctDNA liquid biopsy in accurate screening and early diagnosis of primary liver cancer in cirrhotic population” (No. Z221100007422002), the Capital’s Funds for Health Improvement and Research Project “Prediction model for decompensated hepatitis B cirrhosis after antiviral therapy: a nested case-control study” (No. CFH-2024-2G-2185), and the National Natural Science Foundation of China Project “Evidence-based evaluation and application of intelligent precision surgery for liver cancer” (No. 8209053). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Ethics Committee of Beijing Youan Hospital (approval No. [2018]015) and was implemented following the Declaration of Helsinki and its subsequent amendments. The waiver of informed consent was granted by the hospital Ethics Committee due to the retrospective use of clinical samples in this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rumgay H, Arnold M, Ferlay J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol 2022;77:1598-606. [Crossref] [PubMed]
- Yang JD, Hainaut P, Gores GJ, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 2019;16:589-604. [Crossref] [PubMed]
- Benson AB, D'Angelica MI, Abbott DE, et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021;19:541-65. [Crossref] [PubMed]
- Piñero F, Dirchwolf M, Pessôa MG. Biomarkers in Hepatocellular Carcinoma: Diagnosis, Prognosis and Treatment Response Assessment. Cells 2020;9:1370. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Electronic address: wheeler@bcm; . Comprehensive and Integrative Genomic Characterization of Hepatocellular Carcinoma. Cell 2017;169:1327-1341.e23. [Crossref] [PubMed]
- Wu L, Quan W, Luo Q, et al. Identification of an Immune-Related Prognostic Predictor in Hepatocellular Carcinoma. Front Mol Biosci 2020;7:567950. [Crossref] [PubMed]
- Cheng J, Li Y, Wang X, et al. Response Stratification in the First-Line Combined Immunotherapy of Hepatocellular Carcinoma at Genomic, Transcriptional and Immune Repertoire Levels. J Hepatocell Carcinoma 2021;8:1281-95. [Crossref] [PubMed]
- Peeters F, Cappuyns S, Piqué-Gili M, et al. Applications of single-cell multi-omics in liver cancer. JHEP Rep 2024;6:101094. [Crossref] [PubMed]
- Jin Y, Lee WY, Toh ST, et al. Comprehensive analysis of transcriptome profiles in hepatocellular carcinoma. J Transl Med 2019;17:273. [Crossref] [PubMed]
- Ghosh S, Zhao X, Alim M, et al. Artificial intelligence applied to 'omics data in liver disease: towards a personalised approach for diagnosis, prognosis and treatment. Gut 2025;74:295-311. [Crossref] [PubMed]
- Wang Y, Song F, Zhang X, et al. Mitochondrial-Related Transcriptome Feature Correlates with Prognosis, Vascular Invasion, Tumor Microenvironment, and Treatment Response in Hepatocellular Carcinoma. Oxid Med Cell Longev 2022;2022:1592905. [Crossref] [PubMed]
- Liang J, Chen W, Ye J, et al. Single-cell transcriptomics analysis reveals intratumoral heterogeneity and identifies a gene signature associated with prognosis of hepatocellular carcinoma. Biosci Rep 2022;42:BSR20212560. [Crossref] [PubMed]
- Liu JF, Huang L, Zhou XP, et al. Disulfidptosis and ferroptosis related genes predict prognosis and personalize treatment for hepatocellular carcinoma. Transl Cancer Res 2024;13:496-514. [Crossref] [PubMed]
- Mao J, Song F, Zhang Y, et al. Development and validation of a chromatin regulator signature for predicting prognosis hepatocellular carcinoma patient. J Gastrointest Oncol 2024;15:397-414. [Crossref] [PubMed]
- Li X, Li L, He N, et al. Pathomics signatures and cuproptosis-related genes signatures for prediction of prognosis in patients with hepatocellular carcinoma. Transl Cancer Res 2024;13:5473-83. [Crossref] [PubMed]
- Liu Q, Gu L, Qiu J, et al. Elevated NDC1 expression predicts poor prognosis and correlates with immunity in hepatocellular carcinoma. J Gastrointest Oncol 2023;14:245-64. [Crossref] [PubMed]
- Fu S, Debes JD, Boonstra A. DNA methylation markers in the detection of hepatocellular carcinoma. Eur J Cancer 2023;191:112960. [Crossref] [PubMed]
- Nagaraju GP, Dariya B, Kasa P, et al. Epigenetics in hepatocellular carcinoma. Semin Cancer Biol 2022;86:622-32. [Crossref] [PubMed]
- Campani C, Imbeaud S, Couchy G, et al. Circulating tumour DNA in patients with hepatocellular carcinoma across tumour stages and treatments. Gut 2024;73:1870-82. [Crossref] [PubMed]
- Wu X, Li J, Gassa A, et al. Circulating tumor DNA as an emerging liquid biopsy biomarker for early diagnosis and therapeutic monitoring in hepatocellular carcinoma. Int J Biol Sci 2020;16:1551-62. [Crossref] [PubMed]
- Garcia-Lezana T, Lopez-Canovas JL, Villanueva A. Signaling pathways in hepatocellular carcinoma. Adv Cancer Res 2021;149:63-101. [Crossref] [PubMed]
- Riley RD, Ensor J, Snell KIE, et al. Calculating the sample size required for developing a clinical prediction model. BMJ 2020;368:m441. [Crossref] [PubMed]
- Zhao Q, Wang F, Chen YX, et al. Comprehensive profiling of 1015 patients' exomes reveals genomic-clinical associations in colorectal cancer. Nat Commun 2022;13:2342. [Crossref] [PubMed]
- He Y, Song L, Wang H, et al. Mutational Profile Evaluates Response and Survival to First-Line Chemotherapy in Lung Cancer. Adv Sci (Weinh) 2021;8:2003263. [Crossref] [PubMed]
- Wu B, Yang J, Qin Z, et al. Prognosis prediction of stage IV colorectal cancer patients by mRNA transcriptional profile. Cancer Med 2022;11:4900-12. [Crossref] [PubMed]
- Meng J, Wang F, Ji L, et al. Comprehensive methylation profile of CSF cfDNA revealed pathogenesis and diagnostic markers for early-onset Parkinson's disease. Epigenomics 2021;13:1637-51. [Crossref] [PubMed]
- Nair SS, Luu PL, Qu W, et al. Guidelines for whole genome bisulphite sequencing of intact and FFPET DNA on the Illumina HiSeq X Ten. Epigenetics Chromatin 2018;11:24. [Crossref] [PubMed]
- Guo DZ, Zhang X, Zhang SQ, et al. Single-cell tumor heterogeneity landscape of hepatocellular carcinoma: unraveling the pro-metastatic subtype and its interaction loop with fibroblasts. Mol Cancer 2024;23:157. [Crossref] [PubMed]
- Midorikawa Y, Yamamoto S, Tatsuno K, et al. Accumulation of Molecular Aberrations Distinctive to Hepatocellular Carcinoma Progression. Cancer Res 2020;80:3810-9. [Crossref] [PubMed]
- Peng X, Zhu J, Liu S, et al. Signature construction and molecular subtype identification based on cuproptosis-related genes to predict the prognosis and immune activity of patients with hepatocellular carcinoma. Front Immunol 2022;13:990790. [Crossref] [PubMed]
- Cho HJ, Kim SS, Wang HJ, et al. Detection of Novel Genomic Markers for Predicting Prognosis in Hepatocellular Carcinoma Patients by Integrative Analysis of Copy Number Aberrations and Gene Expression Profiles: Results from a Long-Term Follow-Up. DNA Cell Biol 2016;35:71-80. [Crossref] [PubMed]
- Yang S, Ko M, Hur SC, et al. SF3B4 Regulates Cellular Senescence and Suppresses Therapy-induced Senescence of Cancer Cells. Cancer Genomics Proteomics 2024;21:622-9. [Crossref] [PubMed]
- Yan K, Zhang D, Chen Y, et al. Chromosome 11q13 amplification correlates with poor response and prognosis to PD-1 blockade in unresectable hepatocellular carcinoma. Front Immunol 2023;14:1116057. [Crossref] [PubMed]
- Rebouissou S, Nault JC. Advances in molecular classification and precision oncology in hepatocellular carcinoma. J Hepatol 2020;72:215-29. [Crossref] [PubMed]
- Zhang BH, Yang J, Jiang L, et al. Development and validation of a 14-gene signature for prognosis prediction in hepatocellular carcinoma. Genomics 2020;112:2763-71. [Crossref] [PubMed]
- Lu J, Chen Y, Zhang X, et al. A novel prognostic model based on single-cell RNA sequencing data for hepatocellular carcinoma. Cancer Cell Int 2022;22:38. [Crossref] [PubMed]
- Long J, Wang A, Bai Y, et al. Development and validation of a TP53-associated immune prognostic model for hepatocellular carcinoma. EBioMedicine 2019;42:363-74. [Crossref] [PubMed]
- Pallerla SR, Hoan NX, Rachakonda S, et al. Custom gene expression panel for evaluation of potential molecular markers in hepatocellular carcinoma. BMC Med Genomics 2022;15:235. [Crossref] [PubMed]
- Liu J, Sun G, Pan S, et al. The Cancer Genome Atlas (TCGA) based m(6)A methylation-related genes predict prognosis in hepatocellular carcinoma. Bioengineered 2020;11:759-68. [Crossref] [PubMed]
- Li D, Li K, Zhang W, et al. The m6A/m5C/m1A Regulated Gene Signature Predicts the Prognosis and Correlates With the Immune Status of Hepatocellular Carcinoma. Front Immunol 2022;13:918140. [Crossref] [PubMed]
- Xu RH, Wei W, Krawczyk M, et al. Circulating tumour DNA methylation markers for diagnosis and prognosis of hepatocellular carcinoma. Nat Mater 2017;16:1155-61. [Crossref] [PubMed]
- Péneau C, Imbeaud S, La Bella T, et al. Hepatitis B virus integrations promote local and distant oncogenic driver alterations in hepatocellular carcinoma. Gut 2022;71:616-26. [Crossref] [PubMed]


