
Metastatic pancreatic acinar cell carcinoma with BRCA2 gene alternation resected after modified FORFIRINOX therapy: a case report and literature review
Highlight box
Key findings
• Pancreatic acinar cell carcinoma (PACC) can be sensitive to a fluoropyrimidine or a platinum drug; thus, FORFIRINOX (FFX) [modified FFX (mFFX)], not gemcitabine (GEM), could be the first-choice drug for PACC.
What is known and what is new?
• There is currently no standard chemotherapy regimen for PACC; instead, the treatment regimens for pancreatic ductal adenocarcinoma are generally used for PACC.
• PACC was considerably shrunk by mFFX.
• Our patient had a breast cancer susceptibility gene 2 alternation.
What is the implication, and what should change now?
• When managing PACC patients with anti-tumor agents, the treatment might be better to start with FFX (mFFX), rather than GEM plus ablaxan. Further, we should perform cancer genomic profiling and analyze the gene alternation to select the optimal anti-cancer drugs.
Introduction
Pancreatic acinar cell carcinoma (PACC) is a rare subtype of pancreatic cancer, accounting for 0.3–4.3% of all exocrine pancreatic neoplasms (1). The prognosis is considered to be better than that of pancreatic ductal adenocarcinoma (PDAC), with a 5-year survival rate of 21.5–42.8% (1), but its clinicopathological behavior is not fully understood because of its rarity. If curative resection is possible, surgical resection is considered to be the best first-line treatment approach for PACC (2). Further, the conversion surgery for unresectable tumors (locally advanced and metastatic cases) after treatment with an anti-tumor agent reportedly is better to achieve a longer prognosis (3). Thus, effective anti-tumor agents for PACC are necessary. However, there is no standard chemotherapy regimen for PACC; instead, the treatment regimens for PDAC are generally used for PACC. We experienced a case of PACC in a patient with breast cancer susceptibility gene (BRCA)2 alternation whose hepatic tumor considerably shrunk after treatment with modified FORFIRINOX (mFFX). After the mFFX treatment, resection was also possible for this case. An appropriate chemotherapy regimen personalized for each patient is necessary. We present this article in accordance with the CARE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-845/rc).
Case presentation
A 67-year-old man who was treated for breast cancer in 2016 experienced continuous left back pain and visited Kure Medical Center in 2022. Abdominal computed tomography (CT) revealed a 47-mm hypo-dense mass in the pancreatic tail, requiring further examination. He underwent a mastectomy for the left breast cancer and a transurethral resection of the bladder tumor in 2016. Abdominal CT was performed annually to monitor for any relapse of breast cancer. He had hypertension and hyperlipidemia. The patient did not smoke or drink alcohol. His father died from cholangiocarcinoma. Physical examination results on admission were as follows: height, 170 cm; weight, 64 kg; and body temperature, 36.4 °C. His abdomen was soft and flat with no palpable mass. His relevant laboratory results were as follows: amylase, 62 IU/L; lipase, 2,738 IU/L; and elastase-1, 906 ng/dL. The carbohydrate antigen 19-9 and S-pancreas-1 antigen levels were 41 IU/L and 152 U/mL, respectively.
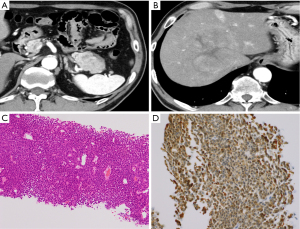
CT revealed a 47-mm hypo- or iso-dense mass with a cystic component in the pancreatic tail (Figure 1A) and a 100-mm round mass with slightly enhanced compared to liver and a cystic component in early phase at segment 8 of the liver (Figure 1B). We performed fine needle aspiration with endoscopic ultrasonography and percutaneous liver biopsy. Histopathological examination of the specimens obtained from both masses revealed atypical cells with swollen nuclei and eosinophilic granular cytoplasm with scant vascular interstitium and sheet-like proliferation in a scattered rosette-like arrangement (Figure 1C). Immunohistochemical examination of the tumor revealed B-cell lymphoma/leukemia 10 (BCL10) positivity (Figure 1D), leading a diagnosis of PACC.
We decided to administer anti-tumor agents to the patient and started with a mFFX regimen as the first-line chemotherapy because of the following reasons: that there were more reports that FFX (mFFX) was effective for PACC, as compared to GEM; and the patient was likely to have a BRCA gene alternation as he had a history of breast cancer. Concurrently, we performed an analysis of BRCA gene alternation with blood and genetic screening (FoundationOne CDx, Chugai Pharmaceutical Co., Ltd., Tokyo, Japan) using a liver biopsy specimen. Both tests identified the germline BRCA2 gene alternation; hence, chemotherapy with mFFX was continued. In the liver biopsy sample, NRAS Q61H gene mutation was also detected.
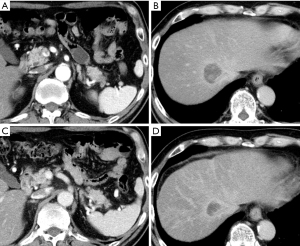
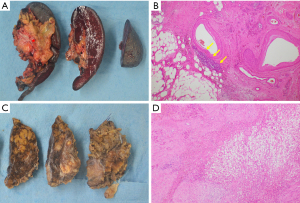
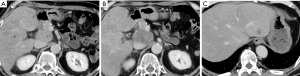
After five cycles of mFFX, the size of the pancreatic and hepatic tumors decreased to 24 mm (Figure 2A) and 32 mm (Figure 2B), respectively. After 13 treatment cycles, the pancreatic and hepatic tumors were 10 mm (Figure 2C) and 24 mm (Figure 2D), respectively, in size. Consequently, he underwent a distal pancreatectomy and a partial hepatectomy of segment 8. The resected specimen showed a residual tumor with a size of approximately 900 µm in the pancreatic tail (effect 3) (Figure 3A,3B), but no residual tumor was noted in the liver (effect 4) (Figure 3C,3D).
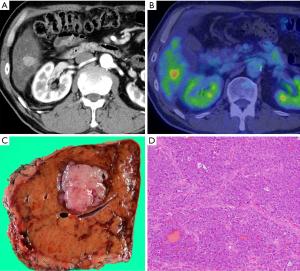
Afterward, he received tegafur-gimeracil-oteracil potassium (S1) for 6 months as an adjuvant therapy. At 18 months after his first visit to our department (at 12 months after surgery), a 30-mm hepatic metastasis was found in segment 6 (Figure 4). He received three cycles of mFFX. As CT revealed a stable disease (SD), he then underwent partial hepatectomy (Figure 4). At 3 months after the second surgery, he was started on olaparib due to the presence of a relapse in the lymph nodes (8p, 13a) (Figure 5). At 3 months after treatment with olaparib, the metastasis had expanded, and olaparib was considered to be inefficacious.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration and its subsequent amendments. Written informed consent for publication of this case report and accompanying images was obtained from the patient. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
We report the case of a patient with PACC who had a BRCA2 gene alternation, in whom conversion surgery was possible owing to the considerable tumor reduction by mFFX therapy. Our case report is valuable, as it recommends multimodal therapy for PACC.
PACC is a tumor originating from the acinar cells of the pancreas and occupied 0.2–4.3% in pancreatic exocrine tumors. The characteristics of PACC are rather different from those of PDAC, and the prognosis of PACC is considered to be better than that of PDAC. However, the 5-year survival rate of PACC is not very high; thus, it is considered a relatively aggressive tumor. Approximately 50% of patients with PACC have a distant metastasis at the time of diagnosis, and the recurrence rate after radical resection could reach as high as 72% (3). From the abovementioned facts, multimodal therapies, including chemotherapy regimens, are needed for PACC (1,3,4).
Our case was metastatic disease and excision of the hepatic metastasis was difficult because of the small remnant liver volume after excision even if we tried. Thus, chemotherapy was selected for the patient. Currently, the standard therapy for PACC has not been established yet and the chemotherapy regimens for PDAC are recommended for PACC. In Japan, GEM plus nab-paclitaxel (GnP) and FFX (mFFX) are recommended as the first-line therapies (5-7). In these two regimens, more patients, especially elderly, tend to receive GnP than FFX (mFFX), because of the weaker side effects of GnP. There were more reports that FFX (mFFX) was effective for PACC, as compared to GEM (Table S1). Thus, we need to reconsider the first-line chemotherapy for PACC based not only on its side effects but also on its effectiveness.
Fluoropyrimidine-based regimen, especially FFX (mFFX), was reported to be more effective than GEM-based regimens (1,3,8-10). Additionally, our male patient had a breast cancer and was suspected of having a BRCA gene alternation. Thus, we started the chemotherapy treatment with mFFX including fluorouracil and oxaliplatin because a patient with a BRCA gene alternation was considered to be sensitive to platinum-based chemotherapy drugs (1,4,11-13). During the treatment course, the presence of BRCA2 gene alternation was determined by centrifugation with blood and liver biopsy samples, and treatment with mFFX was continued, resulting in a considerable tumor shrinkage. The BRCA2 gene is used for DNA repair and the tumor with a BRCA2 gene alternation is sensitive to platinum-based anti-cancer drugs, which inhibit DNA repair. Furthermore, the effectiveness of fluoropyrimidine for PACC is considered to be based on the theory of adenomatous polyposis coli (APC)/β-catenin pathway sensitivity observed in pancreatic acinar cells (8,14,15).
We found 53 PACC cases treated with chemotherapies after a database search in PubMed using the keywords “acinar cell carcinoma” and “case report” from January 2003 to September 2024 (Table S1). We analyzed 54 cases including our case. Altogether, 84 anti-tumor therapies, excluding treatments with molecular-targeted agents, were found and analyzed. Fifty-two treatment regimens that were first initiated for the patients are shown in Table 1. The response rate was significantly higher in the FFX-treated (mFFX-treated) (68.8%) and S1-treated (100%) patients than in the GEM-treated (0%) and GnP-treated (0%) patients. These results suggest that FFX (mFFX) and S1 are more effective than GEM-based chemotherapy. Additionally, some cases had complete remission and partial response (PR) with 5-fluorouracil (5-FU) treatment only (16). Although there have been case reports of partial and complete responses with combination therapy including GEM [GEM + oxaliplatin (17), GEM + S1 (18), GEM + mitomycin (19)], these effects might be induced by other drugs, except for GEM. For irinotecan (IRI), treatment regimens utilizing IRI only were not reported; however, four treatment regimens including IRI except for mFFX (FFX) have been described in previous reports (2,20-22). A GEM plus IRI regimen (20) resulted in progressive disease (PD), and a treatment with cisplatin plus IRI (2) resulted in SD. Two cases receiving IRI hydrochloride hydrate plus 5-FU (21,22) and folic acid showed PR and PD. Although Takahashi et al. (9) have reported that IRI-based agents were favored for PACC, on the basis of the abovementioned data, we considered that IRI might not be very effective. On conversion surgery after chemotherapy in patients with unresectable PACC, fluoropyrimidine-based regimen, especially FFX (mFFX), had shown favorable result (3,12,16,22-30).
Table 1
| Regimen | First line | Second line | All | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | RR | DCR | Number | RR | DCR | Number | RR | DCR | |||
| FFX (mFFX) | 16 | 11/68.8 | 14/87.5 | 3 | 3/100 | 3/100 | 19 | 14/73.7 | 17/89.4 | ||
| GEM | 12 | 0/0 | 5/33.3 | 1 | 0/0 | 0/0 | 13 | 0/0 | 4/30.8 | ||
| GnP | 5 | 0/0 | 1/20 | 5 | 0/0 | 0/0 | 10 | 0/0 | 1/10 | ||
| S1 | 3 | 3/100 | 3/100 | 5 | 1/20 | 1/20 | 8 | 4/50 | 4/50 | ||
| GEMOX | 2 | 2/100 | 2/100 | 2 | 0/0 | 1/50 | 4 | 2/50 | 3/75 | ||
| GEM plus S1 | 2 | 2/100 | 2/100 | – | – | – | 2 | 2/100 | 2/100 | ||
| Cisplatin plus IRI | 1 | 1/100 | 1/100 | – | – | – | 1 | 1/100 | 1/100 | ||
| FOLFOX | 1 | 0/0 | 1/100 | 3 | 2/66.7 | 2/66.7 | 4 | 2/50 | 3/75 | ||
| CAPTEM | 1 | 0/0 | 0/0 | – | – | – | 1 | 0/0 | 0/0 | ||
| XEROX | 1 | 1/100 | 1/100 | – | – | – | 1 | 1/100 | 1/100 | ||
| GEM plus cisplatin | 1 | 1/100 | 1/100 | – | – | – | 1 | 1/100 | 1/100 | ||
| Paclitaxel | 1 | 0/0 | 0/0 | 1 | 1/100 | 1/100 | 2 | 1/50 | 1/50 | ||
| 5-FU | 1 | 1/100 | 1/100 | – | – | – | 1 | 1/100 | 1/100 | ||
| GEM plus IRI | 1 | 0/0 | 1/100 | – | – | – | 1 | 0/0 | 1/100 | ||
| GEM plus mitomycin | 1 | 1/100 | 1/100 | – | – | – | 1 | 1/100 | 1/100 | ||
| 5-FU plus cisplatin | 1 | 0/0 | 1/100 | – | – | – | 1 | 0/0 | 1/100 | ||
| Nal-IRI | – | – | – | 2 | 1/50 | 1/50 | 2 | 1/50 | 1/50 | ||
| SOX | – | – | – | 1 | 0/0 | 0/0 | 1 | 0/0 | 0/0 | ||
| FORFIRI | – | – | – | 4 | 2/50 | 2/50 | 4 | 2/50 | 2/50 | ||
| Carboplatin | – | – | – | 1 | 0/0 | 1/100 | 1 | 0/0 | 1/100 | ||
| GEM plus 5-FU | – | – | – | 1 | 1/100 | 1/100 | 1 | 1/100 | 1/100 | ||
| Docetaxel plus capecitabine | – | – | – | 1 | 0/0 | 0/0 | 1 | 0/0 | 0/0 | ||
| Capecitabine | – | – | – | 1 | 0/0 | 0/0 | 1 | 0/0 | 0/0 | ||
| CAPOXIRI | – | – | – | 1 | 0/0 | 0/0 | 1 | 0/0 | 0/0 | ||
| Total | 51 | – | – | 32 | – | – | 83 | – | – | ||
Data in RR and DCR are presented as number/%. 5-FU, 5-fluorouracil; CAPOXIRI, capecitabine, raltitrexed with oxaliplatin, and irinotecan; CAPTEM, capecitabine plus temozolomide; DCR, disease control rate; FFX, FORFIRINOX; FOLFOX, folinic acid, fluorouracil, and oxaliplatin; FORFIRI, folinic acid, fluorouracil, and irinotecan; GEM, gemcitabine; GEMOX, gemcitabine plus oxaliplatin; GnP, gemcitabine plus nab-paclitaxel; IRI, irinotecan; mFFX, modified FORFIRINOX; nal-IRI, folinic acid, fluorouracil, and nanoliposomal irinotecan; PACC, pancreatic acinar cell carcinoma; RR, response rate; SOX, S1 and oxaliplatin; XEROX, capecitabine and oxaliplatin.
In PACC, the alternation of the BRCA1/2 gene was reported to be high as compared with that in PDAC. In particular, the rate of BRCA2 gene alternation reportedly was 13.6–36.7% (PDAC: 2.7–2.9%) (1,31). On the basis of the abovementioned facts, we might need to select treatment regimens that include platinum and fluoropyrimidine for PACC as the first-choice treatment. Under insurance, we should select the FFX (mFFX) as the first regimen.
Patients with a BRCA gene alternation are considered to be sensitive to the platinum-based anti-tumor agents and poly (adenosine diphosphate-ribose) polymerase (PARP) inhibitors (32). The PARP inhibitor acts through multiple mechanisms, including the trapping of PARP on the DNA at the sites of single-strand breaks. In our literature search for cases of PACC with BRCA gene alternation (Table 2), the PARP inhibitor had shown favorable effect. Thus, we need to analyze the gene alternation of germline and somatic BRCA1/2 in PACC patients.
Table 2
| Case No. | DOI (ref.) | Author | Reported year | Age (years old) | Gender (M/F) | Altered gene | Reason for chemotherapy | First line chemotherapy | Effect | Clinical course |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10.1007/s10689-024-00390-3 (11) | Matsubayashi | 2024 | 73 | M | BRCA2 | Liver metastasis in 13 months after surgery | Olaparib | PR | Not available |
| 2 | 10.1002/cnr2.70007 (13) | Kubo | 2024 | 53 | M | BRCA1 | Liver metastasis after surgery | GnP | PD | PR with mFFX in19 cycles. Switch to olaparib (PD in 4 months). PD with mFFX. PD with GEM |
| 3 | 10.1007/s12328-024-01981-4 (4) | Urabe | 2024 | 80 | M | BRCA2 | Relapse in remnant pancreas after surgery | GnP | PD | PD with S1 in 8 months. Radiation. PD with S1 in 5 months. PR with mFFX (continued a year and 3 months). Switch to olaparib (maintained PR in a year). Survival in a year after stopping olaparib |
| 4 | 10.1007/s12328-024-01992-1 (30) | Watanabe | 2024 | 60 | M | BRCA1 | Locally advanced | GnP | PD | PR with FFX in 6 cycles. Sent for conversion surgery. FFX as adjuvant chemotherapy in 6 months. No recurrence in 10 months after stopping FFX |
| 5 | 10.3748/wjg.v28.i45.6421 (33) | Lee | 2022 | 70 | M | BRCA2 | Liver metastasis | mFFX | PR | PR with mFFX in 15 cycles. Switch to olaparib due to peripheral neurological disorders. Maintained PR in 5 months |
| 6 | 10.1002/ccr3.6718 (34) | Lelong | 2022 | 38 | M | BRCA2 | Liver metastasis | FFX | PR | PR with FFX in 12 cycles. Switch to olaparib (PR in 24 months) |
| 7 | 10.1159/000515267 (22) | Dreikhausen | 2021 | 66 | M | BRCA2 | Liver metastasis | FFX | PR | PR with FFX. Exchange to FORFIRI by peripheral neurological disorders by FFX. Sent for conversion surgery. FORFIRI, GnP, and nal-IRI had PD for relapse in the liver. Olaparib was sent but the efficacy was not available |
| 8 | 10.1080/15384047.2019.1595274 (18) | Kryklyva | 2019 | 52 | M | BRCA2 | Metastasis for liver, lung, peritoneum, and skin in 6 months after surgery | FFX | PR | PR with FFX in 24 months. Stopped FFX due to peripheral neurological disorders. Death in 3 months |
| 9 | 10.1097/MD.0000000000013113 (35) | Li | 2018 | 59 | M | BRCA2 | Retroperitoneum lymph node metastasis | FFX | PD | Radiation for lymph-node metastasis (not effective). Intra-tumoral brachytherapy by radioiodine-125 (not effective). PR with olaparib. Stopped olaparib due to severe complications. PD and death |
| 10 | 10.1080/17843286.2016.1168065 (36) | Naeyaert | 2016 | 59 | M | BRCA2 | Liver metastasis | GEM | PD | SD by carboplatin |
| Our case | – | – | – | 67 | M | BRCA2 | Liver metastasis | mFFX | PR | PR with mFFX in 13 cycles. Sent for conversion surgery. Relapse in the liver in 12 months after surgery. SD with FFX in 3 months. Excision liver tumor. Relapse in lymph-node and started olaparib in 3 months after surgery. PD with olaparib |
BRCA, breast cancer susceptibility gene; F, female; FFX, FORFIRINOX; FORFIRI, folinic acid, fluorouracil, and irinotecan; GEM, gemcitabine; GnP, gemcitabine plus nab-paclitaxel; M, male; mFFX, modified FORFIRINOX; nal-IRI, folinic acid, fluorouracil, and nanoliposomal irinotecan; PACC, pancreatic acinar cell carcinoma; PD, progressive disease; PR, partial response; ref., reference; SD, stable disease.
PACC and PDAC are known to have a different gene alternation profile (1,31,37). PDAC has a higher frequency of gene alternation in KRAS, TP53, and CDKN2A (PACC vs. PDAC: 13.6% vs. 85.1%, 15.9% vs. 69.1%, and 25.0% vs. 35.4%, respectively) and lower frequency of gene alternation in BRCA1, BRCA2, BRAF, mismatch repair-deficient (dMMR)/microsatellite instability, and tumor mutational burden-high (PACC vs. PDAC: 2.3% vs. 0.9%, 13.6% vs. 2.9%, 15.9% vs. 1.7%, 2.6% vs. 0.3%, and 7.9% vs. 1.8%, respectively) (1). Additionally, PACC is more likely to have germline and somatic gene alternations as compared to other solid tumors (33). Recently, several reports have described the effectiveness of molecular-targeted drug for PACC (Table 3). Thus, it is essential that we analyze the gene alternation of the tumor. For improvement of the prognosis of patients with PACC, first, a surgery may need to be performed. Even not at the time of the first diagnosis, conversion surgery should be the aim of treatment. During this time, FFX (mFFX) including the administration of a fluoropyrimidine and a platinum-based anti-tumor agent might be the best regimen. Additionally, multimodal approaches can be used, as analyzed in 54 cases describing 15 cases of surgeries, four cases of arterial injection chemotherapy, three cases of radiofrequency ablation, and one case of radiation. Furthermore, many treatment regimens using anti-tumor drugs have been tried (Tables 1-3 and Table S1), which sometimes obtained a response. Thus, patients with PACC might need to be treated through a multimodal approach. Unfortunately, our patient showed disease progression, at the time of writing this manuscript, but we were confident that we might prolong his life by conversion surgery and chemotherapy. Regrading of this point, the effectiveness of resecting PACC with distant metastases and recurrent PACC has not been determined. We need further analysis which therapeutic strategy was useful between chemotherapy only and resecting oligometastatic lesions. FFX, even mFFX, could induce some strong side effects and be difficult to continue for some patients, especially elderly people. A literature search revealed some PACC cases that were sensitive to 5-FU, capecitabine, 5-FU + cisplatin, FOLFOX (folinic acid, fluorouracil, and oxaliplatin), XEROX (capecitabine and oxaliplatin), and SOX (S1 and oxaliplatin). We desire to use these agents under the national insurance coverage in Japan.
Table 3
| Case No. | DOI (ref.) | Author | Reported year | Age (years old) | Gender (M/F) | Mutated gene | Metastatic lesion or locally advanced | Clinical course before using molecular target drug | Molecular target drug | Anti-tumor effect |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10.1007/s00432-024-05841-z (12) | Merz | 2024 | 69 | M | MLH1/MSI-high | Locally advanced | SD with mFFX (6 cycles). Switch to GnP (PD) | Pembrolizumab (anti-PD-1 antibody) | PR. Sent for conversion surgery |
| 2 | 10.1002/gcc.23222 (38) | Von Fritsch | 2024 | 52 | M | BRAFV600E | Peritoneum | SD with mFFX (8 cycles). Exchange to 5-FU plus folic acid due to peripheral neurological disorders. Stopped due to fatigue | Dabrafenib/trametinib (BRAF inhibitor/MEK inhibitor) | CR in 16 months |
| 3 | 10.12998/wjcc.v11.i24.5823 (39) | Wang | 2023 | 77 | M | ROS1-CENPW | Liver | N/A | GnP plus crizotinib (specific tyrosine kinase inhibitor) | PR |
| 4 | 10.3389/fonc.2024.1357233 (24) | Wu | 2024 | 45 | F | High TMB | Locally advanced | N/A | Tripalimab (anti-PD-1 antibody) | PR |
| 5 | 10.1200/PO.21.00400 (21) | Gaule | 2022 | 62 | M | Rearranged ALK | Liver metastasis after surgery | PD with GnP (3 cycles), nal-IRI (PR but PD in 6 months). PD with FOLFOX (7 cycles) | Alectinib (ALK tyrosine kinase inhibitor) | PR in 16 months |
| 6 | 10.6004/jnccn.2020.7641 (40) | Gupta | 2021 | 81 | M | NTRK fusion gene | Lymph-node relapse after surgery | PD with GEM (5 cycles). PD with GnP (4 cycles) | Larotrectinib (NTRK inhibitor) | CR in 13 months |
| 7 | 10.1177/0300891620980792 (41) | Xu | 2020 | 68 | M | PD-L1 positive plus high TMB | Lung, retroperitoneum lymph-node | SD with GnP (2 cycles) and skin rash. PD with SOX (2 cycles) | SOX plus tripalimab (anti-PD-1 antibody) | PR |
| 8 | 10.3389/fonc.2021.692480 (42) | Qin | 2021 | 48 | F | PD-L1 positive plus high TMB | Liver | None | Sintilimab plus lenvatinib | PR |
| 9 | 10.4251/wjgo.v10.i4.103 (43) | Richard | 2018 | 54 | M | EGFR | Liver | PD with FFX (4 cycles). PD with GnP (2 cycles) | Panitumumab (anti-EGFR) | PR |
| 10 | 10.1177/030089161309900230 (44) | Ang | 2013 | 71 | M | N/A | Liver | PR with GEM plus cisplatin (6 months). Renal failure. PD with GEM plus oxaliplatin. PR with FORFIRI (1 year). PR with HAI (floxuridine) plus irinotecan. Irinotecan plus cetuximub (1 year). PD with FORFIRI plus cetuximub | FORFIRI plus cetuximub plus bevacizumab | PR |
| 11 | N/A (20) | Antonie | 2007 | 44 | F | N/A | Liver | SD with GEM plus irinotecan (7 cycles). SD with GEMOX (16 cycles). PD with docetaxel plus capecitabine (6 cycles). PD with FORFIRI | GEM plus erlotinib | PD |
| 12 | 10.1385/IJGC:34:2-3:067 (45) | Pimenta | 2003 | 43 | F | N/A | Peritoneum relapse after surgery | SD with GEM (7 cycles). PD with capecitabine. PD with Raltitrexed plus oxaliplatin. PD with irinotecan. PR with paclitaxel (4 months) | Imatinib mesylate | PD |
5-FU, 5-fluorouracil; BRCA, breast cancer susceptibility gene; CR, complete response; F, female; FOLFOX, folinic acid, fluorouracil, and oxaliplatin; FORFIRI, folinic acid, fluorouracil, and irinotecan; FFX, FORFIRINOX; GEM, gemcitabine; GEMOX, gemcitabine plus oxaliplatin; GnP, gemcitabine plus nab-paclitaxel; HAI, hepatic arterial infusion; M, male; mFFX, modified FORFIRINOX; MSI, microsatellite instability; nal-IRI, folinic acid, fluorouracil, and nanoliposomal irinotecan; N/A, not available; PACC, pancreatic acinar cell carcinoma; PD, progressive disease; PR, partial response; ref., reference; SD, stable disease; SOX, S1 and oxaliplatin; TMB, tumor mutational burden.
Conclusions
PACC seem more sensitive to FFX (mFFX) and even milder fluoropyrimidine-based regimens rather than gemcitabine (GEM) of GnP. Genomic sequencing may provide treatable targets. Surgery may be beneficial in selected oligometastatic cases if notable response to neoadjuvant therapy is seen.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-845/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-845/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-845/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration and its subsequent amendments. Written informed consent for publication of this case report and accompanying images was obtained from the patient. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ikezawa K, Urabe M, Kai Y, et al. Comprehensive review of pancreatic acinar cell carcinoma: epidemiology, diagnosis, molecular features and treatment. Jpn J Clin Oncol 2024;54:271-81. [Crossref] [PubMed]
- Maehira H, Iida H, Mori H, et al. Pathological complete response in a patient with metastatic pancreatic acinar cell carcinoma who received a chemotherapy regimen containing cisplatin and irinotecan. Clin J Gastroenterol 2021;14:1772-8. [Crossref] [PubMed]
- Uemura S, Maeda H, Tanioka N, et al. Successful conversion surgery after FOLFIRINOX therapy in a patient with advanced pancreatic acinar cell carcinoma with a solitary peritoneal dissemination: A case report. Cancer Rep (Hoboken) 2022;5:e1648. [Crossref] [PubMed]
- Urabe M, Ikezawa K, Kozumi K, et al. Long-term survival after systemic chemotherapy, chemoradiotherapy, and maintenance therapy for an older adult patient with recurrent pancreatic acinar cell carcinoma. Clin J Gastroenterol 2024;17:771-5. [Crossref] [PubMed]
- Okusaka T, Ikeda M, Fukutomi A, et al. Phase II study of FOLFIRINOX for chemotherapy-naïve Japanese patients with metastatic pancreatic cancer. Cancer Sci 2014;105:1321-6. [Crossref] [PubMed]
- Ozaka M, Ishii H, Sato T, et al. A phase II study of modified FOLFIRINOX for chemotherapy-naïve patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol 2018;81:1017-23. [Crossref] [PubMed]
- Ueno H, Ikeda M, Ueno M, et al. Phase I/II study of nab-paclitaxel plus gemcitabine for chemotherapy-naive Japanese patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol 2016;77:595-603. [Crossref] [PubMed]
- Xu JY, Guan WL, Lu SX, et al. Optimizing Chemotherapy of Pancreatic Acinar Cell Carcinoma: Our Experiences and Pooled Analysis of Literature. Clin Med Insights Oncol 2022;16:11795549221090186. [Crossref] [PubMed]
- Takahashi H, Ikeda M, Shiba S, et al. Multicenter Retrospective Analysis of Chemotherapy for Advanced Pancreatic Acinar Cell Carcinoma: Potential Efficacy of Platinum- and Irinotecan-Containing Regimens. Pancreas 2021;50:77-82. [Crossref] [PubMed]
- Busch E, Werft W, Bougatf N, et al. Metastatic Acinar Cell Carcinoma of the Pancreas: A Retrospective Cohort Study on Systemic Chemotherapy and Review of the Literature. Pancreas 2021;50:300-5. [Crossref] [PubMed]
- Matsubayashi H, Todaka A, Tsushima T, et al. The response of pancreatic acinar cell carcinoma to platinum and olaparib therapy in a germline BRCA2 variant carrier: case report and literature review. Fam Cancer 2024;23:393-8. [Crossref] [PubMed]
- Merz V, Maines F, Marcucci S, et al. Complete pathological response to pembrolizumab in pretreated pancreatic acinar cell carcinoma. J Cancer Res Clin Oncol 2024;150:347. [Crossref] [PubMed]
- Kubo T, Ikeda Y, Muramatu J, et al. Germline BRCA1-Mutated Synchronous and Metachronous Pancreatic Acinar Cell Carcinoma With Long-Term Survival. Cancer Rep (Hoboken) 2024;7:e70007. [Crossref] [PubMed]
- Hashimoto M, Hikichi T, Suzuki T, et al. Successful chemotherapy with modified FOLFIRINOX for pancreatic acinar cell carcinoma. Clin J Gastroenterol 2017;10:564-9. [Crossref] [PubMed]
- Abraham SC, Wu TT, Hruban RH, et al. Genetic and immunohistochemical analysis of pancreatic acinar cell carcinoma: frequent allelic loss on chromosome 11p and alterations in the APC/beta-catenin pathway. Am J Pathol 2002;160:953-62. [Crossref] [PubMed]
- Distler M, Rückert F, Dittert DD, et al. Curative resection of a primarily unresectable acinar cell carcinoma of the pancreas after chemotherapy. World J Surg Oncol 2009;7:22. [Crossref] [PubMed]
- Béchade D, Desjardin M, Salmon E, et al. Pancreatic Acinar Cell Carcinoma. Case Rep Gastroenterol 2016;10:174-80. [Crossref] [PubMed]
- Kryklyva V, Haj Mohammad N, Morsink FHM, et al. Pancreatic acinar cell carcinoma is associated with BRCA2 germline mutations: a case report and literature review. Cancer Biol Ther 2019;20:949-55. [Crossref] [PubMed]
- Kolb-van Harten P, Rosien U, Klöppel G, et al. Pancreatic acinar cell carcinoma with excessive alpha-fetoprotein expression. Pancreatology 2007;7:370-2. [Crossref] [PubMed]
- Antoine M, Khitrik-Palchuk M, Saif MW. Long-term survival in a patient with acinar cell carcinoma of pancreas. A case report and review of literature. JOP 2007;8:783-9. [PubMed]
- Gaule M, Pesoni C, Quinzii A, et al. Exceptional Clinical Response to Alectinib in Pancreatic Acinar Cell Carcinoma With a Novel ALK-KANK4 Gene Fusion. JCO Precis Oncol 2022;6:e2100400. [Crossref] [PubMed]
- Dreikhausen L, Schulte N, Belle S, et al. Pancreatic Acinar Cell Carcinoma with Germline BRCA2 Mutation and Severe Pancreatic Panniculitis: A Case Report. Visc Med 2021;37:447-50. [Crossref] [PubMed]
- Jimbo M, Batista PM, Baliff JP, et al. Neoadjuvant Chemotherapy and Appleby Procedure for Pancreatic Acinar Cell Carcinoma: A Case Report. Case Rep Pancreat Cancer 2016;2:46-9. [Crossref] [PubMed]
- Wu G, Fang Y, Bi D, et al. Case report: Immunotherapy in rare high TMB pancreatic acinar carcinoma. Front Oncol 2024;14:1357233. [Crossref] [PubMed]
- Izumo W, Higuchi R, Furukawa T, et al. A case of pathologically complete response after preoperative chemotherapy in a pancreatic acinar cell carcinoma patient with portal vein tumor thrombosis. Clin J Gastroenterol 2022;15:642-8. [Crossref] [PubMed]
- Villano AM, Barrak D, Jain A, et al. Robot-assisted combined pancreatectomy/hepatectomy for metastatic pancreatic acinar cell carcinoma: case report and review of the literature. Clin J Gastroenterol 2020;13:973-80. [Crossref] [PubMed]
- Toda H, Kurahara H, Maemura K, et al. A Case of Curative Resection for Advanced Pancreatic Acinar Cell Carcinoma with Liver Metastasis and Involvement of the Superior Mesenteric Artery after Chemoradiotherapy Following Systemic Chemotherapy. Gan To Kagaku Ryoho 2016;43:2071-3. [PubMed]
- Jauch SF, Morris VK, Jensen CT, et al. Multimodal approach and long-term survival in a patient with recurrent metastatic acinar cell carcinoma of the pancreas: A case report. Pancreatology 2016;16:153-6. [Crossref] [PubMed]
- Yamamoto T, Ohzato H, Fukunaga M, et al. Acinar cell carcinoma of the pancreas: a possible role of S-1 as chemotherapy for acinar cell carcinoma. A case report. JOP 2012;13:87-90. [PubMed]
- Watanabe T, Nagaoka Y, Kimura N, et al. A case of BRCA1-mutated giant pancreatic acinar cell carcinoma successfully treated with modified FOLFIRINOX therapy and radical resection. Clin J Gastroenterol 2024;17:970-5. [Crossref] [PubMed]
- Furukawa T, Sakamoto H, Takeuchi S, et al. Whole exome sequencing reveals recurrent mutations in BRCA2 and FAT genes in acinar cell carcinomas of the pancreas. Sci Rep 2015;5:8829. [Crossref] [PubMed]
- Golan T, Hammel P, Reni M, et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med 2019;381:317-27. [Crossref] [PubMed]
- Lee CL, Holter S, Borgida A, et al. Germline BRCA2 variants in advanced pancreatic acinar cell carcinoma: A case report and review of literature. World J Gastroenterol 2022;28:6421-32. [Crossref] [PubMed]
- Lelong M, Raoul JL, Touchefeu Y, et al. Prolonged response on olaparib maintenance in metastatic pancreatic acinar cell carcinoma associated with a germline BRCA 2 mutation, revealed by severe panniculitis. Clin Case Rep 2022;10:e6718. [Crossref] [PubMed]
- Li M, Mou Y, Hou S, et al. Response of germline BRCA2-mutated advanced pancreatic acinar cell carcinoma to olaparib: A case report. Medicine (Baltimore) 2018;97:e13113. [Crossref] [PubMed]
- Naeyaert C, de Clerck F, De Wilde V. Pancreatic panniculitis as a paraneoplastic phenomenon of a pancreatic acinar cell carcinoma. Acta Clin Belg 2016;71:448-50. [Crossref] [PubMed]
- Chmielecki J, Hutchinson KE, Frampton GM, et al. Comprehensive genomic profiling of pancreatic acinar cell carcinomas identifies recurrent RAF fusions and frequent inactivation of DNA repair genes. Cancer Discov 2014;4:1398-405. [Crossref] [PubMed]
- von Fritsch L, von Bubnoff N, Weber K, et al. Near complete remission of an inoperable pancreatic acinar cell carcinoma after BRAF-/MEK-inhibitor treatment-A case report and review of the literature. Genes Chromosomes Cancer 2024;63:e23222. [Crossref] [PubMed]
- Wang T, Shen YY. Rare ROS1-CENPW gene in pancreatic acinar cell carcinoma and the effect of crizotinib plus AG chemotherapy: A case report. World J Clin Cases 2023;11:5823-9. [Crossref] [PubMed]
- Gupta M, Sherrow C, Krone ME, et al. Targeting the NTRK Fusion Gene in Pancreatic Acinar Cell Carcinoma: A Case Report and Review of the Literature. J Natl Compr Canc Netw 2021;19:10-5. [Crossref] [PubMed]
- Xu H, Wang X, Zhou S, et al. Efficacy of chemotherapy combined with toripalimab in PD-L1-positive and high tumor mutation burden pancreatic acinar cell carcinoma: case report. Tumori 2021;107:NP24-7. [Crossref] [PubMed]
- Qin L, Shen J, Yang Y, et al. Rapid Response to the Combination of Lenvatinib and Sintilimab in a Pancreatic Acinar Cell Carcinoma Patient With Elevated Alpha-Fetoprotein: A Case Report. Front Oncol 2021;11:692480. [Crossref] [PubMed]
- Richard C, Niogret J, Boidot R, et al. EGFR amplification induces sensitivity to antiEGFR therapy in pancreatic acinar cell carcinoma. World J Gastrointest Oncol 2018;10:103-7. [Crossref] [PubMed]
- Ang C, Herran LA, Lagunes DR, et al. A case report of a patient with advanced acinar cell carcinoma of the pancreas: long-term survival with regional, systemic and targeted therapy. Tumori 2013;99:e61-4. [Crossref] [PubMed]
- Riechelmann RP, Hoff PM, Moron RA, et al. Acinar cell carcinoma of the pancreas. Int J Gastrointest Cancer 2003;34:67-72. [Crossref] [PubMed]


