
Lacticaseibacillus casei 393 modulates KRAS and APC expression and cytokine levels in colitis-associated colon cancer
Highlight box
Key findings
• In the murine azoxymethane-dextran sodium sulfate (AOM-DSS) model of colitis-associated colon cancer (CAC): (I) Lacticaseibacillus casei 393 (L. casei 393) decreased the apparition of preneoplastic lesion in colon and the levels of pro-inflammatory cytokines; (II) L. casei 393 modulated Kristen rat viral sarcoma oncogene homolog (KRAS) and adenomatosis polyposis coli gene (APC) expression.
What is known, and what is new?
• L. casei 393 is a lactic acid probiotic with anti-inflammatory properties that delay colon carcinogenesis. KRAS and the APC are key genes for colon carcinogenesis.
• L. casei 393 modulated colon inflammation. Its administration at the same time as CAC induction with AOM-DSS decreased KRAS oncogene expression, and its administration alone or before AOM-DSS increased APC tumor suppressor gene expression in colon of BALB/c mice.
What is the implication, and what should change now?
• The present pre-clinical work supports the therapeutic potential of L. casei 393 against CAC.
• The moderate consumption of probiotic strains in CAC patients to target KRAS-induced damaging effects on the immune system is promising.
• More investigation on KRAS and APC down-pathways will be addressed to get a comprehensive view of the molecular mechanisms that provide L. casei 393 its properties.
Introduction
Colorectal cancer (CRC) is the third most diagnosed cancer worldwide and the second cause of cancer-related death, representing 10% of the global cancer incidence. Due to population aging and unfavorable lifestyles, the incidence of CRC will likely increase in many countries in the following decades (1-3). Colitis-associated colon cancer (CAC) is a specific subset of CRC that affects patients with chronic inflammatory bowel diseases (IBDs), including Crohn’s disease and ulcerative colitis. IBD affects the gastrointestinal tract and progressively destroys the intestinal architecture (4). Chronic inflammation is also mutagenic, and depending on the duration and severity of the IBD, it may increase CAC’s prevalence (5). Overall, the risk of colon cancer rises between 1.5 and 2.4 times among IBD patients compared to the general population (3). Furthermore, although CAC accounts for only 1–2% of CRC (3,5), it represents one of the most severe complications of IBD and is responsible for the death of approximately 10–15% of IBD patients (3,6,7).
The classic Fearon-Vogelstein adenoma-carcinoma sequence provides a general step-by-step model for colon tumorigenesis, which associates the accumulation of genetic alterations with evolving colon histopathology (8,9). However, even if CAC and CRC share many pathological similarities, these diseases present variations in the timing and frequency of their genetic alterations (5-7). At the tissue level, gene alterations enable the low-to-high-grade dysplasia transition of colon epithelial cells, the progressive formation of polyps (small benign adenoma), early adenoma (large benign adenoma), late adenoma, and carcinoma, which may advance to cancer and metastasize (1). The Fearon-Vogelstein model highlights the critical role of mutations that activate the Kristen rat viral sarcoma oncogene homolog (KRAS) proto-oncogene or inactivate the adenomatosis polyposis coli (APC) tumor-suppressor gene. Altered expression of these genes participates in the initiation and progression of colon cancer (5-9). KRAS is a G-protein that acts as a molecular switch, cycling between inactive (Guanosine diphosphate-bound) and active (Guanosine triphosphate-bound) states. Mutational activation of its coding gen maintains KRAS’s active form, resulting in malignant transformation and uncontrolled cell growth, cancer initiation, and progression. Mutations in the KRAS gene are amongst the most ubiquitous oncogenic drivers in human cancers (9-12). According to Li et al. (13), the mutation frequency of KRAS is 41% among colon cancer patients; thus, KRAS is a therapeutic target for colon cancer treatment. On the other hand, biallelic loss of APC also contributes to colon cancer. As part of the APC complex, this tumor-suppressor protein participates in the proteasome-mediated degradation of β-catenin in the cytoplasm, avoiding its translation to the nucleus and the activation of the Wnt/β-catenin signaling pathway involved in cellular processes such as loss of cell differentiation, cell survival and hyperproliferation (13,14).
Moreover, colon cancer involves multiple environmental, immunologic, and microbial factors (6,7). The tumor microenvironment (TME) includes tumoral cells, immune cells, fibroblasts, and endothelial cells. It can present pro- or antitumoral effects in response to different factors, such as persistent infections or inflammation, that activate the innate immune system as a primary response (15). Although the precise role of immune cells in the immediate inflammatory response is incompletely understood, it is clear that the tumor exploits the dynamic changes in the TME. The tumor-promoting pathway starts with continuous inflammation, triggering immune signals such as the secretion of cytokines from the tumor and the host cells (16). Nascent malignant cells develop mechanisms to escape immune surveillance, such as loss of antigenicity and immunogenicity; tumors may also build an immunosuppressive response by secreting cytokines that prevent the immune response and complicate the elimination of tumoral cells (17,18).
The interplay between diet and gut microbiota is closely associated with the incidence and development of CAC; it is a factor that patients can easily and inexpensively modify to prevent or fight the disease (19,20). A healthy intestinal microbiota enhances the gut’s immune capacity to avoid infection; dysbiosis, however, results in inflammatory diseases that provoke carcinogenesis by altering the immune response and the epithelial integrity. Lactobacillus spp is a group of lactic acid probiotic microorganisms and a natural functional food source. Beyond their nutritional value, these microorganisms may also provide health benefits. Lacticaseibacillus casei is found naturally in our digestive system and has a significant role in intestinal tract health; this microorganism is also known to alleviate multiple diseases, although its potential depends on the strain, dose, and treatment duration (21-24). Some valuable properties of lactic acid bacteria against colon cancer onset and progression are the induction of cell apoptosis, their antioxidant activity, and the modulation of the host immune response (25-28). Heydari et al. also reported the production of antimicrobial substances and bacteriocins (29). Probiotics may also avoid the dysregulation of tight junction proteins (occludin, claudins, junctional adhesion molecules), preventing damage to the epithelial barrier and protecting the digestive system from intestinal inflammation (19). Altogether, these properties help preserve or restore the balance between beneficial and detrimental gut microbiota for proper gut homeostasis.
Despite the important progress in CAC treatment, this disease is still a leading life-threatening malignancy, and patients’ management is not yet at a satisfactory level. Probiotics are known to play an essential role in CAC prevention or treatment. Thus, the present study used the CAC model induced in BALB/c mice with azoxymethane (AOM) and dextran sulfate sodium (DSS) to assess the effect of the administration of the probiotic strain Lacticaseibacillus casei ATCC 393 (L. casei 393) on colon histopathology, circulating cytokines, and colon KRAS and APC mRNA expression. We present this article in accordance with the ARRIVE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-667/rc).
Methods
Reagents and chemicals
AOM was obtained from Sigma (St. Louis, MO, USA). Colitis-grade DSS, with a molecular weight range of 36 to 50 KDa, was from MP Biomedical (Solon, OH, USA). Hematoxylin and eosin (H&E) were purchased from Sigma (St. Louis, MO, USA). TRIzol, the Oligo dT12-18 primers, and the Murine-Maloney-leukemia-virus reverse transcriptase (M-MLV-RT) were from Invitrogen Life Technology (Carlsbad, CA, USA). The quantitative polymerase chain reaction (qPCR) master mix (Maxima SYBR-Green/ROX) was purchased from Thermo Scientific (Waltham, MA, USA), and the Man-Rogosa-Sharpe (MRS) broth was from Difco (Becton Dickinson, MD, USA). All other chemicals were molecular biology grade.
Probiotic strain
The Lacticaseibacillus casei 393 (27) strain was purchased from the American Type Culture Collection (ATCC, USA) and was cultivated in MRS at 37 ℃ under aerobic conditions.
AOM and DSS treatment
Mouse models of chemically induced CAC, particularly the AOM-DSS model, are powerful tools for understanding the disease and testing therapeutic agents. AOM acts as a specific colon carcinogen that initiates the neoplastic process by alkylating DNA, which facilitates mismatch pairing of nitrogenous bases; DSS is a pro-colitis agent that causes epithelial barrier dysfunction, increases the permeability of the colonic mucosa and promotes chronic inflammation (30). The induction of CAC in BALB/c mice was through two intraperitoneal (i.p.) injections of the carcinogen AOM (10 mg/kg body weight), with a seven-day interval between the two injections. One week after the second AOM injection, the animals were administered DSS (2% w/v) in drinking water for 5 days. Three cycles of 2% DSS were given to the animals with a one-week rest between cycles.
Experimental groups
Thirty-five, 6-week-old BALB/c female mice (Mus musculus), weighing 17 to 18 g, were included in this study based on previous work from our research group (31-33). The animals were obtained from the National Institute of Public Health in Cuernavaca, Morelos, Mexico. All experimental procedures were planned to minimize animal suffering, and the total number of animals used. Mice were kept in optimum animal housing conditions at a controlled temperature of 22±1 ℃, with light/dark cycles of 12 h. The animals had access to standard commercial rodent food and water ad libitum. The mice were acclimated for 2 weeks and then randomly separated into five groups (7 animals/group, 3 to 4 animals/cage, 2 cages/group) as follows: Control group: healthy animals that received no treatment; AOM-DSS group: animals treated with AOM and DSS (CAC control group); LC (L. casei 393) group: animals that received L. casei for 24 weeks without AOM-DSS treatment; LCP (L. casei 393 preventive) group: animals given L. casei four weeks before the AOM-DSS treatment and continuing for 24 weeks; LCA (L. casei 393 at the same time) group: animals given L. casei for 24 weeks, starting at the same time as AOM-DSS (Figure 1). The weight of the animals was registered weekly. Outcomes were assessed after six months of treatment start. The animals were euthanized, and blood samples were rapidly collected by cardiac puncture to measure serum cytokines. Distal colon segments were immediately dissected for histopathological and gene expression evaluations. Animals were excluded if they died prematurely, preventing the collection of the biological samples. Some mice blood samples were not analyzed for cytokine levels due to insufficient blood drawn. A protocol was prepared before the study with registration in the Cellular and Molecular Biology Department and Research Coordination of our campus at the University of Guadalajara. This protocol conformed to the Rules for Research in Health Matters (Mexican Official Norms NOM-062-ZOO-1999) (34) for the care and use of animals. It was approved by the institutional ethics committee of the Biological and Agricultural Sciences Campus of the University of Guadalajara (approval number CINV-C/033/2024).
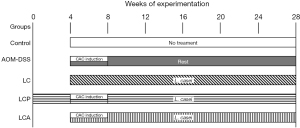
Lacticaseibacillus casei ATCC 393 administration
The probiotic was administered by oral gavage at the dose of 1×106 CFU/100 µL in phosphate-buffered saline (PBS 1X, pH 7.4) per mouse twice a week (once a day) (31-32) for 24 weeks (LC and LCA groups) or 28 weeks (LCP group).
Colon histopathological examination
The formalin fixed paraffin embedded colon tissues (n=7 per experimental group) were sectioned, and the sections (5 µm) were stained with H&E and observed under a light microscope (AxiostarPlus, Zeiss, Oberkochen, Germany) under blind experimental conditions using a 40× objective.
Evaluation of circulating cytokines
Following the manufacturer’s instructions, serum cytokines were quantified using the LEGENDPlexTM mouse inflammation panel kit (Biolegend, San Diego, CA, USA, Cat No 740446). The samples of all experimental groups were stored at −80 ℃. For this analysis, n=5 for each experimental group was used. The Attune Acoustic Focusing Cytometer (Applied Biosystems, Waltham, MA, USA) was used in which 2,100 events (resin Beads Size A) were acquired and analyzed using the Biolegend LEGEND plex v8.0 data analysis software (San Diego, CA, USA).
RNA extraction, cDNA synthesis, and reverse transcription‑quantitative polymerase chain reaction
Each distal colon segment was rapidly weighed and embedded in TRIzol (1 mL TRIzol/100 mg tissue). RNA extraction was performed according to the manufacturer’s protocol. RNA integrity and concentration were evaluated by ultraviolet-spectrophotometry (Nanodrop One spectrophotometer, Thermo Fisher Scientific, Waltham, NA, USA) and 1% agarose gel electrophoresis. cDNAs were synthesized from 500–1,000 ng of total RNA using oligo-dt12-18 and M-MLV reverse-transcriptase according to the manufacturer’s protocol (Invitrogen, Carlsbad, USA) (35). The resulting cDNAs were also quantified by spectrophotometry. Details regarding primer pairs and qPCR conditions have been described elsewhere (33). For this analysis, n=6 was used for each experimental group, and each cDNA sample was evaluated in triplicate. The constitutive housekeeping gene (β-actin) and the genes of interest (GOI) were always assessed on the same plate. The stability of β-actin expression was verified by one-way analysis of variance (ANOVA); no statistical difference in the cycle threshold (Ct) values of β-actin was observed between the healthy control group (16.63±0.38) and tumor group (17.99±0.92). The Ct data of KRAS and APC expression (Ct) were normalized to that of β-actin using the 2−∆∆Ct method to calculate the relative gene expression (36). The Control group was the reference group.
Statistical analysis
Cytokine data are expressed as the mean ± standard error of the mean (SEM). The 2−∆∆Ct data are expressed as mean ± standard deviation (SD). All data analyzed passed the Shapiro-Wilk normality test. One-way ANOVA followed by the comparison of significances between groups with Tukey’s post hoc test were performed using the GraphPad Prism software 8.0.2. The probability level of P<0.05 was considered a statistically significant difference.
Results
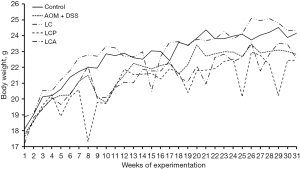
BALB/c mice body weight
The weight of the animals was registered during the experiment, including the acclimation period (Figure 2). The data show that the Control and LC groups presented similar weight curves and, at the end of the experimental period, slightly higher weight than the animals exposed to AOM and DSS (AOM-DSS, LCP, and LCA groups). However, these differences did not reach significance.
Effect of L. casei 393 on distal colon histopathology in the AOM-DSS model of CAC
H&E staining of colonic tissue was performed at the end of the experimental period to validate the induction of CAC in AOM-DSS BALB/c mice and evaluate the effect of L. casei 393 supplementation on colon integrity and CAC process.
The Control group showed the typical colonic architecture. In contrast, the AOM-DSS mice lost colon architecture, showing severe lymphocyte infiltration, adenocarcinomas, and cellular atypia with larger and darker nuclei. L. casei 393 administration alone (LC group) did not significantly change colon architecture and functionality; however, the mice presented goblet cells with hyperplasia and hypertrophy, low-to-moderate inflammation with infiltration of macrophages, neutrophils, and a few lymphocytes into the lamina propria. The LCP group that received L casei 393 4 weeks before AOM-DSS showed functional crypts but moderate macrophage and leukocyte inflammation. In the LCA group, starting the administration of L. casei at the same time as the AOM-DSS, the treatment prevented the formation of adenocarcinomas in all animals, and only one animal presented preneoplastic lesions. The animals from this group showed functional crypts but some infiltration of mononuclear cells (Figure 3).

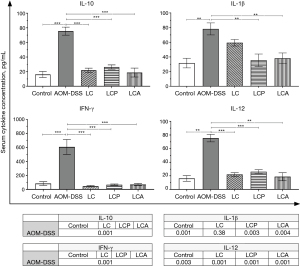
Effect of L. casei 393 on circulating cytokines
The circulating cytokine levels were measured by flow cytometry in the different experimental groups to confirm the inflammatory process associated with our CAC model and evaluate the effect of L. casei 393 in this model.
The levels of the circulating interleukin (IL)-10, IL-1β, IFN-γ, and IL-12 increased significantly in the AOM-DSS group compared to the other groups. However, L. casei 393 ingestion decreased the levels of all four cytokines in the LCA and LCP groups compared to the AOM-DSS group, back to the levels of the healthy Control group. The levels of IL-10, IFN-γ, and IL-12 were similar in the control, LC, LCP, and LCA groups; however, the production of IL-1β tended to increase in the LC group compared to these groups (Figure 4).
Effect of L. casei 393 on KRAS and APC mRNA expression in the colon of BALB/c mice
Quantitative reverse transcription polymerase chain reaction (RT-qPCR) was used to measure the effect of L. casei administration on the transcriptional expression of colonic KRAS and APC in BALB/c mice.
The expression of the KRAS oncogene (mRNA) increased significantly in the AOM-DSS group compared to the Control group (P<0.001). The LC and LCP (P<0.001) groups presented higher levels of KRAS mRNA than the Control and AOM-DSS groups. However, KRAS mRNA levels decreased significantly in the LCA group (P<0.001) compared to the AOM-DSS, LC, and LCP groups, reaching levels like those of the Control group (Figure 5A).

The expression of the APC tumor suppressor gene (mRNA) decreased significantly in the AOM-DSS group compared to the Control group (P=0.01) but increased in the LC group compared to Control and LCP groups (P<0.001). The LCP group presented APC mRNA levels similar to the Control group. The APC expression tended to increase in the LCP group compared to the AOM-DSS group (P=0.01) but without reaching significance. The LCP mice presented an intermediate APC mRNA expression level compared to LC and LCA. Finally, the LCA group demonstrated significantly lower levels of APC mRNA than the Control, LC, and LCP groups (P<0.001) but not compared to the AOM-DSS group (P=0.25) (Figure 5B).
Discussion
In the murine AOM-DSS model of CAC, L. casei decreased the apparition of preneoplastic lesions in the distal colon and circulating pro-inflammatory cytokines levels; L. casei also modulated KRAS and APC colonic expression.
The histopathological assessment of the distal colon sections confirmed that the AOM-DSS treatment successfully induced CAC in the colon of BALB/c mice, which presented adenocarcinoma, loss of colon architecture, and severe infiltration of immune cells, indicative of an inflammatory TME. These features agree with previous reports on murine colon cancer models that mimic the human disease’s histopathological characteristics (6,7,30,37). Interestingly, the ingestion of L. casei 393 counteracted the damaging effects of AOM-DSS in the LCA and LCP groups of mice, significantly reducing colon tissue damage, tumor development, and inflammation compared to the AOM-DSS group. Some mechanisms of action of probiotics, such as L. casei 393, may involve the protection of the intestinal barrier function, the direct inactivation of AOM, or limiting the entry of this carcinogen into the epithelial cells (21,28,29,38,39).
The present report extends previous work from our research group on the effect of L. casei 393 in the 1,2-dimethylhydrazine (DMH) model of sporadic CRC (31,32). Considering that patients with CAC have a worse prognosis than sporadic CRC patients, extending the study of L. casei 393 to a CAC murine model was of relevance. In the DMH model (DMH is the precursor of AOM), we reported that the best effect of L. casei 393 was observed in the LCP group that started probiotic ingestion four weeks before initiating CRC induction. Meanwhile, in the present work, the best effect corresponded to the LCA group that simultaneously initiated L. casei 393 ingestion and CAC induction. This difference may be due to the damaging properties of DSS in the AOM-DSS model, as this pro-colitis agent inflicts damage to the colon epithelium barrier, resulting in a permeability increase and favoring inflammation (37).
Indeed, the levels of pro-inflammatory and anti-inflammatory cytokines (IL-10, IFN-γ, IL-12, IL-1β) drastically increased in the AOM-DSS group. Olsen et al. (40) reported high levels of cytokines (IL-10 and IFN-γ) in the plasma of CRC patients and associated them with poor outcomes and high mortality. Hnatyszyn et al. (4) and Spella et al. (41) also reviewed the principal cytokines in colon cancer (CRC and CAC), reporting the participation of IL-1β as a mediator of cancer-related inflammation. We also detected high levels of IL-12 and IFN-γ in the AOM-DSS group. These data are related since the secretion of IFN-γ mediates the effects of IL-12, which is known to inhibit cancer development (42,43). Our data corresponds to the report of Burgos-Molina et al. (44) in AOM mice exposed to increased levels of DSS (0.1–2%), which presented high tumor incidence, number of tumors per colon, tumoral load, STAT3 phosphorylation (Tyr705), IL-6, tumor necrosis factor α (TNF-α), and IL-12 expression. These cytokines participate in the TME to limit colon cancer growth and avoid angiogenesis; in particular, IL-12 regulates inflammation by connecting innate and adaptive immune responses and exerts antitumoral effects by regulating tumor angiogenesis (4,42).
Interestingly, the ingestion of L. casei 393 decreased the levels of all four cytokines in the LCA and LCP groups compared to the AOM-DSS group, back to the levels of the healthy Control group. Likewise, Silveira et al. (38) described that the administration of Lactobacillus bulgaricus (1×109 CFU/200 µL/three times a week for 12 weeks) inhibited CAC in an AOM-DSS model through negative regulation of intestinal inflammation. Jacouton et al. (28) also reported the beneficial effect of Lactobacillus casei BL23 on the histopathological features of AOM-treated mice. In a DSS-induced model of IBD, Liu et al. (21) reported that the administration of Lactobacillus casei M2S01 for seven days effectively relieved colitis, recovered body weight, and modulated colon cytokine expression compared to DSS alone. These same authors suggested that the anti-inflammatory effect of L. casei M2S01 is related to the inhibition of the NF-κB pathway by hindering the phosphorylation of P65 since p-P65 is directly related to the activation of this pathway. Yu et al. (39) also reported that Lactobacillus plantarum improved DSS-induced gut microbiota dysbiosis, which is known to drive the development of colon cancer. Supplementation with this probiotic significantly reduced the expression of p-P65 and p-IkB (p-KappaB Kinase), indicating that L. plantarum may attenuate colitis by inhibiting the TLR4-MyD88-NF-kB signaling pathways. In agreement with these authors, our experimental data suggest that the positive effect of the L. casei 393 in the AOM-DSS model of CAC may involve similar mechanisms. Additionally, the impact of lactates from lactic acid bacteria through the modulation of the expression of the GPR81 lactate receptor has also been recently reported (45,46).
The RT-qPCR analysis showed higher expression of KRAS mRNAs in the AOM-DSS group compared to the Control group, which agrees with the damaged colon tissue architecture, inflammation, and high circulating cytokines levels observed in these mice. Pereira et al. (12) and Hamarsheh et al. (47) reviewed the oncogenic role of KRAS as a TME modulator in different types of cancers (pancreas, lung, and colon) and reported that KRAS-activating mutations are associated with more tumor aggressiveness and less survival. Thus, in agreement with these reports, our data suggest that in the AOM-DSS model, KRAS overexpression induced an inflammatory TME and poor immune response, facilitating colon tissue damage and carcinogenesis. KRAS expression induces NF-kB signaling and mediates the RAF/MAPK and PI3K intracellular signaling pathways, promoting the transcription of several cytokine genes, including IL-10 and IL-1β (48-50). Additionally, extreme IL-10 levels sustain an immunosuppressive TME through interaction with its receptor and downstream activation of the STAT3 pathway, promoting tumor progression (4,16,51). Thus, it is desirable to evaluate these signaling routes in future work. We also report that the AOM-DSS mice presented low levels of APC tumor-suppressor mRNA compared to the Control group. The synergy between KRAS over-expression and APC down-expression certainly contributed to the aggressiveness of the disease in this group of mice, enhancing Wnt signaling, as reviewed by Jeong et al. (14).
It is necessary to pay special attention to the LCA group in which the administration of L. casei 393 reversed the effect of AOM-DSS on KRAS mRNA expression. This data coincides with colon tissue integrity and normal cytokine levels in this group of mice, suggesting that low KRAS expression avoided excessive cytokine production, particularly IL-10 and IL1β. Heydari et al. (29), in an AOM model, also reported that L. acidophilus and B. bifidum restored the colonic expression of several miRNAs and their target genes, including KRAS and APC. Thus, our work, which focused on KRAS and APC, complements their findings. Conversely, the LC and LCP groups presented signs of colon inflammation (low-to-moderate and moderate, respectively), and high levels of KRAS mRNA at the end of the experimental period, which may be related to an inflammatory effect of the long-term exposure to the probiotic, as suggested by the histopathological evaluation of the LC group, together with the exposure to DSS (in the LCP group), impairing adequate KRAS transcriptional regulation.
Several authors have reviewed the distinct genetic features of sporadic CRC and CAC (6,7,12,52,53) and reported that APC mutations are less frequent and occur later in CAC than in sporadic CRC. For instance, Robles et al. (54) reported that, in colon cancer patients, CAC tumors have a lower prevalence of APC mutations than sporadic CRC tumors. Yaeger et al. (55) also found that the APC genomic alterations were significantly less frequent in CAC than in sporadic CRC. Accordingly, in the present work, we observed that the induction of CAC in the AOM-DSS group had a more substantial effect on KRAS mRNA variations than on APC mRNA variations, expressed as fold changes. More investigation on KRAS and APC protein expression and down-regulated pathways will be undertaken to give a more comprehensive view of the cellular and molecular mechanisms that provide L. casei 393 with the properties described here.
Conclusions
The present work, which focused on evaluating KRAS and APC mRNA expression, circulating cytokine levels, and colon architecture, supports the therapeutic potential of L. casei 393 against CAC. Our data suggest that in the LCA group, this probiotic strain restored cytokine homeostasis and KRAS expression and protected colonocytes from DNA injury. The moderate use of probiotic strains to target KRAS-induced effects on the immune system during CAC onset and progression is promising and deserves further investigation.
Acknowledgments
We thank Karen Alejandra Aguilar-González from the University of Guadalajara for primers design and optimization of RT-qPCR; Xochitl Rocio Ávila-Davila from the University of Guadalajara for technical assistance with the preparation and staining of paraffin sections; Salvador González-Ochoa from the Western Biomedical Research Center of the Mexican Social Security Institute for technical flow cytometry protocol; Luz Patricia Castro-Félix from the University of Guadalajara for commenting on the manuscript and Rebeca Mendez-Hernandez for editing the manuscript in English.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-667/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-667/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-667/prf
Funding: This research was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-667/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under the authorization of the institutional ethics committee of the Biological and Agricultural Sciences Campus
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hossain MS, Karuniawati H, Jairoun AA, et al. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers (Basel) 2022;14:1732. [Crossref] [PubMed]
- Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol 2021;14:101174. [Crossref] [PubMed]
- Maryńczak K, Włodarczyk J, Sabatowska Z, et al. Colitis-Associated Colorectal Cancer in Patients with Inflammatory Bowel Diseases in a Tertiary Referral Center: A Propensity Score Matching Analysis. J Clin Med 2022;11:866. [Crossref] [PubMed]
- Hnatyszyn A, Hryhorowicz S, Kaczmarek-Ryś M, et al. Colorectal carcinoma in the course of inflammatory bowel diseases. Hered Cancer Clin Pract 2019;17:18. [Crossref] [PubMed]
- Li J, Ma X, Chakravarti D, et al. Genetic and biological hallmarks of colorectal cancer. Genes Dev 2021;35:787-820. [Crossref] [PubMed]
- Van Der Kraak L, Gros P, Beauchemin N. Colitis-associated colon cancer: Is it in your genes? World J Gastroenterol 2015;21:11688-99. [Crossref] [PubMed]
- Kameyama H, Nagahashi M, Shimada Y, et al. Genomic characterization of colitis-associated colorectal cancer. World J Surg Oncol 2018;16:121. [Crossref] [PubMed]
- Ciepiela I, Szczepaniak M, Ciepiela P, et al. Tumor location matters, next generation sequencing mutation profiling of left-sided, rectal, and right-sided colorectal tumors in 552 patients. Sci Rep 2024;14:4619. [Crossref] [PubMed]
- Lewis S, Telgenhoff D, Dubansky B. Molecular characterization of colorectal cancers. Clin Lab Sci 2024. Available online:
10.29074/ascls.2022003208 10.29074/ascls.2022003208 - Ciardiello D, Maiorano BA, Martinelli E. Targeting KRAS(G12C) in colorectal cancer: the beginning of a new era. ESMO Open 2023;8:100745. [Crossref] [PubMed]
- Negri F, Bottarelli L, de'Angelis GL, et al. KRAS: A Druggable Target in Colon Cancer Patients. Int J Mol Sci 2022;23:4120. [Crossref] [PubMed]
- Pereira F, Ferreira A, Reis CA, et al. KRAS as a Modulator of the Inflammatory Tumor Microenvironment: Therapeutic Implications. Cells 2022;11:398. [Crossref] [PubMed]
- Li B, Zhang G, Xu X. APC mutation correlated with poor response of immunotherapy in colon cancer. BMC Gastroenterol 2023;23:95. [Crossref] [PubMed]
- Jeong WJ, Ro EJ, Choi KY. Interaction between Wnt/β-catenin and RAS-ERK pathways and an anti-cancer strategy via degradations of β-catenin and RAS by targeting the Wnt/β-catenin pathway. NPJ Precis Oncol 2018;2:5. [Crossref] [PubMed]
- Kather JN, Halama N. Harnessing the innate immune system and local immunological microenvironment to treat colorectal cancer. Br J Cancer 2019;120:871-82. [Crossref] [PubMed]
- Hirano T, Hirayama D, Wagatsuma K, et al. Immunological Mechanisms in Inflammation-Associated Colon Carcinogenesis. Int J Mol Sci 2020;21:3062. [Crossref] [PubMed]
- Galluzzi L, Buqué A, Kepp O, et al. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol 2017;17:97-111. [Crossref] [PubMed]
- Shah SC, Itzkowitz SH. Colorectal Cancer in Inflammatory Bowel Disease: Mechanisms and Management. Gastroenterology 2022;162:715-730.e3. [Crossref] [PubMed]
- Loke YL, Chew MT, Ngeow YF, et al. Colon Carcinogenesis: The Interplay Between Diet and Gut Microbiota. Front Cell Infect Microbiol 2020;10:603086. [Crossref] [PubMed]
- Taghinezhad-S S, Mohseni AH, Fu X. Intervention on gut microbiota may change the strategy for management of colorectal cancer. J Gastroenterol Hepatol 2021;36:1508-17. [Crossref] [PubMed]
- Liu Y, Li Y, Yu X, et al. Physiological Characteristics of Lactobacillus casei Strains and Their Alleviation Effects against Inflammatory Bowel Disease. J Microbiol Biotechnol 2021;31:92-103. [Crossref] [PubMed]
- Asadi Z, Abbasi A, Ghaemi A, et al. Investigating the properties and antibacterial, antioxidant, and cytotoxicity activity of postbiotics derived from Lacticaseibacillus casei on various gastrointestinal pathogens in vitro and in food models. GMS Hyg Infect Control 2024;19:Doc60. [PubMed]
- Rivero García PC, Monroy-Torres R. The effect of probiotics in the treatment and prevention of metabolic syndrome: a systematic review. Mexican Journal of Eating Disorders 2022;12:71-87.
- Behbahani BA, Jooyandeh H, Taki M, et al. Evaluation of the probiotic, anti-bacterial, anti-biofilm, and safety properties of Lacticaseibacillus paracasei B31-2. LWT 2024;207:116676. [Crossref]
- Qin D, Ma Y, Wang Y, et al. Contribution of Lactobacilli on Intestinal Mucosal Barrier and Diseases: Perspectives and Challenges of Lactobacillus casei. Life (Basel) 2022;12:1910. [Crossref] [PubMed]
- Rocha-Ramírez LM, Pérez-Solano RA, Castañón-Alonso SL, et al. Probiotic Lactobacillus Strains Stimulate the Inflammatory Response and Activate Human Macrophages. J Immunol Res 2017;2017:4607491. [Crossref] [PubMed]
- Zheng J, Wittouck S, Salvetti E, et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol 2020;70:2782-858. [Crossref] [PubMed]
- Jacouton E, Chain F, Sokol H, et al. Probiotic Strain Lactobacillus casei BL23 Prevents Colitis-Associated Colorectal Cancer. Front Immunol 2017;8:1553. [Crossref] [PubMed]
- Heydari Z, Rahaie M, Alizadeh AM, et al. Effects of Lactobacillus acidophilus and Bifidobacterium bifidum Probiotics on the Expression of MicroRNAs 135b, 26b, 18a and 155, and Their Involving Genes in Mice Colon Cancer. Probiotics Antimicrob Proteins 2019;11:1155-62. [Crossref] [PubMed]
- Venkatachalam K, Vinayagam R, Arokia Vijaya Anand M, et al. Biochemical and molecular aspects of 1,2-dimethylhydrazine (DMH)-induced colon carcinogenesis: a review. Toxicol Res (Camb) 2020;9:2-18. [Crossref] [PubMed]
- Irecta-Nájera CA, Del Rosario Huizar-López M, Casas-Solís J, et al. Protective Effect of Lactobacillus casei on DMH-Induced Colon Carcinogenesis in Mice. Probiotics Antimicrob Proteins 2017;9:163-71. [Crossref] [PubMed]
- Casas-Solís J, Huizar-López MDR, Irecta-Nájera CA, et al. Immunomodulatory Effect of Lactobacillus casei in a Murine Model of Colon Carcinogenesis. Probiotics Antimicrob Proteins 2020;12:1012-24. [Crossref] [PubMed]
- Huizar-López M, Santerre A, Coronilla-Martínez J, et al. Annona muricata ethanolic extract protects BALB/c mice against colitis-associated colon cancer through modulation of cytokine levels and KRAS and APC expression. Adv Tradit Med 2024. Available online:
10.1007/s13596-024-00798-3 10.1007/s13596-024-00798-3 - Secretary of Agriculture, Livestock, Rural Development, Fisheries and Food, Official Federal Gazette, Official Mexican Standard (NOM), NOM-62-ZOO-199, Mexico 2001.
- Lorenzana-Martínez G, Santerre A, Andrade-González I, et al. Effects of Hibiscus sabdariffa calyces on spatial memory and hippocampal expression of BDNF in ovariectomized rats. Nutr Neurosci 2022;25:670-80. [Crossref] [PubMed]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402-8. [Crossref] [PubMed]
- Yao D, Dong M, Dai C, et al. Inflammation and Inflammatory Cytokine Contribute to the Initiation and Development of Ulcerative Colitis and Its Associated Cancer. Inflamm Bowel Dis 2019;25:1595-602. [Crossref] [PubMed]
- Silveira DSC, Veronez LC, Lopes-Júnior LC, et al. Lactobacillus bulgaricus inhibits colitis-associated cancer via a negative regulation of intestinal inflammation in azoxymethane/dextran sodium sulfate model. World J Gastroenterol 2020;26:6782-94. [Crossref] [PubMed]
- Yu P, Ke C, Guo J, et al. Lactobacillus plantarum L15 Alleviates Colitis by Inhibiting LPS-Mediated NF-κB Activation and Ameliorates DSS-Induced Gut Microbiota Dysbiosis. Front Immunol 2020;11:575173. [Crossref] [PubMed]
- Olsen RS, Nijm J, Andersson RE, et al. Circulating inflammatory factors associated with worse long-term prognosis in colorectal cancer. World J Gastroenterol 2017;23:6212-9. [Crossref] [PubMed]
- Spella M, Ntaliarda G, Skiadas G, et al. Non-Oncogene Addiction of KRAS-Mutant Cancers to IL-1β via Versican and Mononuclear IKKβ. Cancers (Basel) 2023;15:1866. [Crossref] [PubMed]
- Li P, Zhang H, Ji L, et al. A Review of Clinical and Preclinical Studies on Therapeutic Strategies Using Interleukin-12 in Cancer Therapy and the Protective Role of Interleukin-12 in Hematological Recovery in Chemoradiotherapy. Med Sci Monit 2020;26:e923855. [Crossref] [PubMed]
- Tugues S, Burkhard SH, Ohs I, et al. New insights into IL-12-mediated tumor suppression. Cell Death Differ 2015;22:237-46. [Crossref] [PubMed]
- Burgos-Molina AM, Téllez Santana T, Redondo M, et al. The Crucial Role of Inflammation and the Immune System in Colorectal Cancer Carcinogenesis: A Comprehensive Perspective. Int J Mol Sci 2024;25:6188. [Crossref] [PubMed]
- Ranganathan P, Shanmugam A, Swafford D, et al. GPR81, a Cell-Surface Receptor for Lactate, Regulates Intestinal Homeostasis and Protects Mice from Experimental Colitis. J Immunol 2018;200:1781-9. [Crossref] [PubMed]
- Li X, Yao Z, Qian J, et al. Lactate Protects Intestinal Epithelial Barrier Function from Dextran Sulfate Sodium-Induced Damage by GPR81 Signaling. Nutrients 2024;16:582. [Crossref] [PubMed]
- Hamarsheh S, Groß O, Brummer T, et al. Immune modulatory effects of oncogenic KRAS in cancer. Nat Commun 2020;11:5439. [Crossref] [PubMed]
- Rébé C, Ghiringhelli F. Interleukin-1β and Cancer. Cancers (Basel) 2020;12:1791. [Crossref] [PubMed]
- Kaler P, Augenlicht L, Klampfer L. Macrophage-derived IL-1beta stimulates Wnt signaling and growth of colon cancer cells: a crosstalk interrupted by vitamin D3. Oncogene 2009;28:3892-902. [Crossref] [PubMed]
- Fu X, Wang X, Duanmu J, et al. KRAS mutations are negatively correlated with immunity in colon cancer. Aging (Albany NY) 2020;13:750-68. [Crossref] [PubMed]
- Sullivan KM, Jiang X, Guha P, et al. Blockade of interleukin 10 potentiates antitumour immune function in human colorectal cancer liver metastases. Gut 2023;72:325-37. [Crossref] [PubMed]
- Borowczak J, Szczerbowski K, Maniewski M, et al. The Role of Inflammatory Cytokines in the Pathogenesis of Colorectal Carcinoma-Recent Findings and Review. Biomedicines 2022;10:1670. [Crossref] [PubMed]
- Zhou RW, Harpaz N, Itzkowitz SH, et al. Molecular mechanisms in colitis-associated colorectal cancer. Oncogenesis 2023;12:48. [Crossref] [PubMed]
- Robles AI, Traverso G, Zhang M, et al. Whole-Exome Sequencing Analyses of Inflammatory Bowel Disease-Associated Colorectal Cancers. Gastroenterology 2016;150:931-43. [Crossref] [PubMed]
- Yaeger R, Shah MA, Miller VA, et al. Genomic Alterations Observed in Colitis-Associated Cancers Are Distinct From Those Found in Sporadic Colorectal Cancers and Vary by Type of Inflammatory Bowel Disease. Gastroenterology 2016;151:278-287.e6. [Crossref] [PubMed]


