
Efficacy and safety of dose adjustment for fruquintinib in the third-line treatment of metastatic colorectal cancer: a retrospective study with real-world settings
Highlight box
Key findings
• We have provided a new treatment plan with adjusted dosing frequency for patients with metastatic colorectal cancer (mCRC) who cannot tolerate the standard dose of fruquintinib.
What is known and what is new?
• In the real-world clinical setting, a subset of patients is intolerant to the standard dose of fruquintinib therapy.
• Our study indicated that the median progression-free survival and median overall survival for patients with mCRC receiving adjusted-dose fruquintinib was comparable to those treated with standard-dose fruquintinib
What is the implication, and what should change now?
• For patients with mCRC who are intolerant to the standard dose of fruquintinib therapy, an adjusted dosing schedule of fruquintinib administration may be considered.
Introduction
Metastatic colorectal cancer (mCRC) is associated with a poor prognosis, exhibiting a 5-year survival rate of approximately 14% (1,2). Patients with mCRC who have failed first-line or second-line treatments are advised to consider regorafenib (3), fruquintinib (4), or Trifluridine and tipiracil (TAS-102) (5) as third-line therapy according to the guidelines established by the Chinese Society of Clinical Oncology (CSCO).
Fruquintinib is a novel and highly selective oral tyrosine kinase inhibitor that predominantly targets vascular endothelial growth factor receptor (VEGFR) 1, VEGFR2, and VEGFR3 to exert its anti-tumor effects on metastasis and neovasculogenesis (6). The randomized FRESCO trial indicated that the median overall survival (mOS) for patients receiving fruquintinib as third-line treatment was 9.3 months, significantly longer than the placebo group’s mOS by 2.7 months. Additionally, the median progression-free survival (mPFS) reached 3.7 months—considerably longer than that observed in the placebo group by 1.9 months—with a corresponding reduction in the risk of disease progression or death by 74% (4). The subsequent FRESCO-2 clinical trial demonstrated that fruquintinib treatment significantly extended the mOS of patients with mCRC compared to the placebo group (7). Clinical guidelines recommend administering fruquintinib at a dose of 5 mg once daily for three weeks followed by one week off within each 28-day cycle.
While fruquintinib can extend survival in patients with mCRC to some extent, the adverse reactions associated with the standard dosing regimen are significant among Chinese patients, leading some individuals experience difficulty in tolerating the related toxic side effects. According to the safety analysis of the FRESCO clinical trial, the most common treatment-related grade ≥3 adverse events included hypertension, hand-foot skin reaction (HFSR), and proteinuria (4,8,9). Therefore, it is crucial to implement personalized treatment strategies and dose adjustments based on the physical condition of Chinese patients in real-world settings. In this study, we retrospectively analyzed a total of 99 mCRC patients who received either frequency-adjusted fruquintinib monotherapy as the third-line therapy. We evaluated both anti-tumor efficacy and clinical safety to enhance available treatment options for mCRC patients who were unable to tolerate standard-dose therapies in clinical practice. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-2024-881/rc).
Methods
Study design and patients
The data of patients with mCRC who received fruquintinib as the third-line therapy between 2020 and 2024 were collected in Nanjing Drum Tower Clinical Cancer Center and analyzed retrospectively. We then enrolled 99 patients who had changed to an adjusted frequency of fruquintinib (5 mg orally for 7 consecutive days within a 14-day treatment cycle) because they could not tolerate the standard frequency. Follow-up data of PFS were unavailable for 7 patients during the treatment course, and these patients were excluded as they constituted a small proportion of the total enrolled patients. The demographic and clinical characteristics of the patients, pathological features, treatment schedules and response, follow-up data about the safety of treatment by medical records, and survival data were obtained from telephone follow-up records. All enrolled patients must meet the following criteria: (I) pathologically confirmed colorectal cancer patients; (II) mCRC patients with measurable lesions according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1; (III) previously treated with standard first-line and second-line therapies; (IV) aged ≥18 years. Patients with other malignancies were excluded. Patients who lost to follow-up were excluded. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study was conducted with the approval of the Ethics Committee of Nanjing Drum Tower Hospital (No. YBK-2018-001-01) and informed consent was taken from all the patients.
Efficacy and safety assessment
During the treatment period, we regularly followed up with patients with reexamined computed tomography (CT) or magnetic resonance imaging (MR) to assess treatment efficacy. In terms of safety and side effects, we regularly followed up on the general condition and laboratory test results of the patients. Telephone follow-up was performed until tumor progression or death or discontinuation of treatment due to intolerable side effects. The tumor response was assessed according to RECIST (version 1.1): complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The objective response rate (ORR; ORR = CR + PR) and the disease control rate (DCR; DCR = CR + PR + SD) were analyzed. Adverse reactions were assessed based on the National Cancer Institute (NCI) Common Toxicity Criteria for Adverse Events (CTCAE) version 5.0
Statistical analyses
Data were analyzed using SPSS version 25.0. Descriptive statistical methods (number, percentage, median, etc.) were used. All patients initially received the standard regimen of oral fruquintinib at the start of third-line treatment. If patients experienced intolerable drug-related adverse events during the first cycle, they were transitioned to an adjusted dosing frequency of fruquintinib. Progression-free survival (PFS) was defined as the interval from the date of the first cycle of the third-line treatment to the date of disease progression. Overall survival is defined as the time from the third-line treatment until death. For patients who were still alive at the planned analysis cutoff, we used their last known date of survival. For patients who have missed follow-up appointments, we use the date of their last visit as the cut-off point. Kaplan-Meier method was used for survival estimates. Categorical comparisons between groups were calculated using Chi-squared tests (Pearson Chi-Square, Continuity Correction, Fisher’s Exact Test). The results were evaluated at the 95% confidence interval and two-sided P<0.05 was considered to indicate statistical significance.
Results
Patients’ characteristics
Between 2020 and 2024, a total of 99 patients were included in this retrospective study, with the cutoff date for PFS and OS set at October 30, 2024. The median age of the patients was 59 years (range: 38–91 years), and males comprised 65.7% (65/99) of the cohort. 25.2% (25/99) of patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0. The primary tumor was located in the right colon in 19 cases (20.0%), in the left colon in 44 cases (44.4%), and in the rectum in 36 cases (36.4%) (Table 1). All patients had mCRC with proficient mismatch repair or microsatellite stability (MSS) subtypes and had received two prior standard treatment regimens. In this study, the 99 patients who received adjusted-frequency fruquintinib treatment all experienced significant toxicities and side effects that were difficult to tolerate during their first cycle of standard fruquintinib therapy. Major adverse effects observed during their initial administration of the standard fruquintinib regimen included elevated alanine aminotransferase or aspartate aminotransferase levels, hypertension, hand-foot skin reaction, and thrombocytopenia.
Table 1
| Characteristics | All (N=99), n (%) | P value |
|---|---|---|
| Sex | 0.70 | |
| Male | 65 (65.6) | |
| Female | 34 (34.3) | |
| Age (years) | 0.92 | |
| ≥65 | 43 (43.4) | |
| <65 | 56 (56.6) | |
| Primary tumor location | 0.53 | |
| Left-side colon | 44 (44.4) | |
| Right-side colon | 19 (19.2) | |
| Rectum | 36 (36.4) | |
| Number of metastatic organs | 0.31 | |
| ≥3 | 24 (24.2) | |
| <3 | 75 (75.8) | |
mCRC, metastatic colorectal cancer.
Treatment
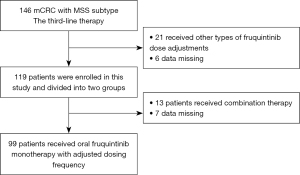
A total of 146 patients received fruquintinib as a third-line treatment. Among them, 21 patients received other types of dose-adjustment treatment with fruquintinib, 6 patients had their follow-up data lost, and 13 patients received a combined treatment mode of fruquintinib and other anti-tumor therapies. Therefore, a total of 99 patients received monotherapy with fruquintinib, and the specific frequency adjustment was to 5 mg orally for 7 consecutive days within a 14-day treatment cycle. Treatment continued until disease progression, intolerable toxicity, patient withdrawal, or death occurred. The screening procedures of the third-line treatment approach for patients with mCRC are illustrated in Figure 1.
Efficacy
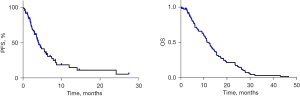
Among the 99 patients received fruquintinib monotherapy. None of the patients achieved CR. The best response evaluations indicated PR in two patients (2.0%), SD in 38 patients (38.4%), and PD in 59 patients (59.6%). As shown in Figure 2, patients receiving fruquintinib at adjusted doses had a mPFS of 4.1 months, and their mOS was 11.4 months. The ORR was 2.0% (2/99), while the DCR was recorded at 40.4%.
Safety
The adverse reactions observed following fruquintinib treatment with an adjusted dosing schedule were relatively less severe compared to those reported in previous studies using standard-dose regimens (8). Overall, 91 out of 99 patients (92.0%) experienced at least one adverse event, including fatigue (21.4%, n=24), HFSR (13.1%, n=13), hypertension (25.3%, n=25), elevated ALT/AST levels (28.3%, n=28), neutropenia (24.2%, n=24), thrombocytopenia (17.2%, n=17), nausea and vomiting (14.2%, n=14), diarrhea (6.1%, n=6). The toxicity was manageable with appropriate symptomatic supportive care, with most events classified as grade 1 to 2 treatment-related adverse reactions; no grade 4 treatment-related adverse reactions or fatalities were reported.
Discussion
The FRESCO clinical trial demonstrated that the median OS for patients with mCRC in the fruquintinib group was 9.30 months, significantly surpassing that of the placebo group, which corresponded to a 35% reduction in mortality risk. Additionally, treatment with fruquintinib resulted in a notable extension of PFS, nearly two months longer than the placebo group (3.71 vs. 1.84 months), and led to a 74% decrease in disease progression risk while significantly enhancing both ORR and DCR. Furthermore, the FRESCO-2 clinical trial corroborated these findings within a larger cohort, confirming that fruquintinib therapy yielded substantial improvements in OS compared to placebo among patients with refractory mCRC (7). Consequently, based on data from both FRESCO and FRESCO-2 trials, fruquintinib has been established as one of the recommended agents for the third-line treatment of mCRC. Notably, fruquintinib is recognized as the first highly selective inhibitor targeting all three VEGF receptor kinases approved for treating mCRC irrespective of biomarker status.
In clinical practice, we have observed that the standard regimen of fruquintinib is associated with significant adverse effects (8-10), leading to a substantial number of Chinese patients being unable to tolerate these side effects. In light of this, the author advocates for a thorough exploration of alternative dosing strategies or administration frequencies for fruquintinib, aiming to establish a more individualized and safer approach for third-line treatment in mCRC. This retrospective study represents the first evaluation of the anti-tumor efficacy and safety of dosing frequency-adjusted fruquintinib in a real-world context for third-line treatment of mCRC. In this study, we modified the dosing frequency of fruquintinib for patients who were intolerant to the standard regimen. We observed improved tolerance among patients following this adjustment. Our findings indicated that for certain mCRC patients who are unable to tolerate the standard dosing frequency, an adjusted regimen can still achieve favorable anti-tumor efficacy, thereby offering a novel perspective on the clinical application of fruquintinib.
However, this study is a retrospective, single-center clinical investigation involving 99 patients. Given the relatively small sample size, this limitation may impact the robustness of our conclusions. To address these limitations and provide more reliable clinical evidence, we are actively conducting prospective clinical trials to explore the three-line comprehensive anti-tumor treatment model for patients with mCRC. Furthermore, as an anti-angiogenic targeted drug, fruquintinib holds potential for combination with immunotherapy and radiotherapy. Recent clinical studies have demonstrated its value in combination therapy settings (11-14). We anticipate that future clinical data will provide further validation of its efficacy in real-world clinical practice.
Conclusions
Despite the limitations inherent in this retrospective study, our findings offer valuable insights into the real-world application of fruquintinib for third-line treatment of mCRC. We contend that appropriate adjustments to the dosing frequency of fruquintinib in selected patients may not significantly compromise anti-tumor efficacy while ensuring safety and tolerability.
Acknowledgments
We sincerely express our gratitude to all the reviewers and editors.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-2024-881/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-2024-881/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-2024-881/prf
Funding: This research was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-2024-881/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study was conducted with the approval of the Ethics Committee of Nanjing Drum Tower Hospital (No. YBK-2018-001-01) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dekker E, Tanis PJ, Vleugels JLA, et al. Colorectal cancer. Lancet 2019;394:1467-80. [Crossref] [PubMed]
- Kumar R, Harilal S, Carradori S, et al. A Comprehensive Overview of Colon Cancer- A Grim Reaper of the 21st Century. Curr Med Chem 2021;28:2657-96. [Crossref] [PubMed]
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303-12. [Crossref] [PubMed]
- Li J, Qin S, Xu RH, et al. Effect of Fruquintinib vs Placebo on Overall Survival in Patients With Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial. JAMA 2018;319:2486-96. [Crossref] [PubMed]
- Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 2015;372:1909-19. [Crossref] [PubMed]
- Chen Z, Jiang L. The clinical application of fruquintinib on colorectal cancer. Expert Rev Clin Pharmacol 2019;12:713-21. [Crossref] [PubMed]
- Dasari A, Lonardi S, Garcia-Carbonero R, et al. Fruquintinib versus placebo in patients with refractory metastatic colorectal cancer (FRESCO-2): an international, multicentre, randomised, double-blind, phase 3 study. Lancet 2023;402:41-53. [Crossref] [PubMed]
- Li J, Guo W, Bai Y, et al. Safety Profile and Adverse Events of Special Interest for Fruquintinib in Chinese Patients with Previously Treated Metastatic Colorectal Cancer: Analysis of the Phase 3 FRESCO Trial. Adv Ther 2020;37:4585-98. [Crossref] [PubMed]
- Deng YY, Zhang XY, Zhu PF, et al. Comparison of the efficacy and safety of fruquintinib and regorafenib in the treatment of metastatic colorectal cancer: A real-world study. Front Oncol 2023;13:1097911. [Crossref] [PubMed]
- Patell K, Mears VL, Storandt MH, et al. Metabolism, toxicity and management of fruquintinib: a novel drug for metastatic colorectal cancer. Expert Opin Drug Metab Toxicol 2024;20:197-205. [Crossref] [PubMed]
- Kroeze SGC, Pavic M, Stellamans K, et al. Metastases-directed stereotactic body radiotherapy in combination with targeted therapy or immunotherapy: systematic review and consensus recommendations by the EORTC-ESTRO OligoCare consortium. Lancet Oncol 2023;24:e121-32. [Crossref] [PubMed]
- Guo Y, Zhang W, Ying J, et al. Phase 1b/2 trial of fruquintinib plus sintilimab in treating advanced solid tumours: The dose-escalation and metastatic colorectal cancer cohort in the dose-expansion phases. Eur J Cancer 2023;181:26-37. [Crossref] [PubMed]
- Wang F, Jin Y, Wang M, et al. Combined anti-PD-1, HDAC inhibitor and anti-VEGF for MSS/pMMR colorectal cancer: a randomized phase 2 trial. Nat Med 2024;30:1035-43. [Crossref] [PubMed]
- Zhao W, Lei J, Ke S, et al. Fecal microbiota transplantation plus tislelizumab and fruquintinib in refractory microsatellite stable metastatic colorectal cancer: an open-label, single-arm, phase II trial (RENMIN-215). EClinicalMedicine 2023;66:102315. [Crossref] [PubMed]


