
Downregulation of CPT2 promotes proliferation and migration through the TNFα/NF-κB pathway in cholangiocarcinoma
Highlight box
Key findings
• Carnitine palmitoyltransferase II (CPT2) expression is positively correlated with the prognosis of cholangiocarcinoma (CCA) patients, and CPT2 can inhibit the progression of cholangiocarcinoma through the inhibition of the TNFα/NF-κB signaling pathway.
What is known and what is new?
• CPT2, a crucial regulatory enzyme in fatty acid oxidation, is associated with the prognosis and progression of numerous cancers.
• This study revealed that CPT2 expression is downregulated in CCA and positively correlates with the prognosis of CCA patients. The overexpression of CPT2 inhibits the TNFα/NF-κB signaling pathway, thereby suppressing the malignant progression of CCA, making it a potential prognostic and therapeutic target for CCA patients.
What is the implication, and what should change now?
• This study demonstrates that CPT2 can predict the prognosis of CCA patients, and modulating the expression of CPT2 can significantly inhibit the malignant progression of CCA cells. Therefore, CPT2 serves as a novel prognostic marker and a potential therapeutic target for CCA patients, and further clinical exploration is necessary.
Introduction
Cholangiocarcinoma (CCA) arises from the malignancy of bile duct epithelial cells owing to various factors; it is highly malignant and has a low survival rate. Currently, surgical resection remains the most effective treatment for CCA, but it is only suitable for patients in the early stage. Factors affecting post-surgical survival include margin condition (1), lymph node metastasis, vascular invasion, and residual liver function (2,3). For patients who cannot receive surgical treatment, the median survival time without treatment is only 6.02 months (4). Radiotherapy/chemotherapy can improve the overall survival rate to some extent, but the median survival time is still only 11.7 months (5). Therefore, improved prognostic markers and new therapeutic targets are needed to increase the overall survival rate of CCA.
Altered lipid metabolism is an important marker for the diagnosis of malignant tumors. In many cancers, the absorption, storage, and production of fat are increased in tumor cells, which promotes their proliferation and metastasis (6). Fat is derived from fatty acids, which are vital for triglyceride synthesis and energy storage. Thus, fatty acid synthesis and oxidation contribute to lipid metabolism, which is closely related to the proliferation, differentiation, migration, and invasion of cancer cells (7). Fatty acid oxidation is an important source of adenosine triphosphate (ATP) and has a role in maintaining cellular energy homeostasis in tumor metabolism (8). Carnitine palmitoyltransferase II (CPT2) is a rate-limiting enzyme involved in fatty acid oxidation. CPT2 deficiency in healthy individuals leads to lipid metabolism disorders and multiple metabolic diseases. However, CPT2 deficiency can promote the malignant progression of tumors. CPT2 expression is downregulated in hepatocellular carcinoma, potentially increasing stearoyl-CoA desaturase-1 (SCD1) expression, a key enzyme involved in monounsaturated fatty acid synthesis; thus, liver cancer cells increase fat production and this accelerates proliferation (9). Furthermore, the expression of CPT2 is down-regulated in other tumors, such as colon cancer (10) and ovarian cancer (11), and is significantly associated with poor prognosis. However, the expression and role of CPT2 in CCA are still unclear and are worthy of further study.
In this study, we investigated CPT2 expression in CCA and its relationship with CCA prognosis and determined whether CPT2 has the potential to be used as a prognostic marker of CCA. An evaluation of CCA prognosis is important for making targeted treatment strategies. Furthermore, our study elucidated the effect of CPT2 on CCA cell function, and deeply explored the molecular mechanism behind this effect, offering new directions for improving the pathogenesis of CCA in the future. We present this article in accordance with the MDAR reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-685/rc).
Methods
Bioinformatics analysis
To explore the expression of CPT2 in CCA, the GSE107943 data set was downloaded from the Gene Expression Omnibus (GEO) database, which included transcriptional profiles of 57 cases of RNA-seqs: 30 cases of CCA tissues and 27 cases of adjacent tissues. The “TCGA-CHOL” dataset was downloaded from The Cancer Genome Atlas (TCGA) database, comprising 45 RNA-seqs transcriptome profiles, including 36 CCA tissues and 9 adjacent tissues. CPT2 gene expression data were extracted from TCGA and GEO data and compared with the expression of CPT2 in CCA and normal tissues. A unified and standardized pan-cancer data set “TCGA Pan-Cancer” was downloaded from the University of California Santa Cruz (UCSC) database, and 10,535 RNA-seqs transcriptome profiles were obtained. CPT2 expression data were extracted from each sample; those with zero expression levels were excluded. Subsequently, cancers with fewer than three samples in a single cancer type were removed, resulting in data from 26 cancers for differential expression analysis. Univariate and multivariate COX regression analyses were used to determine the independent prognostic factors; a nomogram was constructed to visually predict 1-, 3-, and 5-year CCA survival visually. 30 tumor samples from the GEO dataset (GSE107943) were categorized into the high-CPT2-expression group and the low-CPT2-expression group according to the median value of CPT2 expression as cutoff value (cutoff value =5.524058). A Kaplan-Meier curve was generated to assess the relationship between CPT2 expression and CCA survival in the GEO dataset. A time-dependent receiver operating characteristics (ROCs) curve was utilized to evaluate CPT2’s predictive efficacy for 1-, 3-, and 5-year CCA survival. Furthermore, the “h.all.v7.5.1.symbols.gmt” gene set was downloaded from the Molecular Signatures Database, and Gene Set Enrichment Analysis (GSEA) v.4.2.3 was applied to analyze GEO datasets to elucidate the molecular mechanism of CPT2. One thousand permutations were performed to calculate the normalized enrichment score (NES), normal P value, and false discovery rate (FDR q value). The correlation between CPT2 and related genes or proteins was determined using the cBioPortal database.
Patient sample collection
In this study, 11 pairs of fresh CCA tissues and adjacent normal tissues were collected from The Second Affiliated Hospital of Kunming Medical University (Kunming, China; from December 2021 to April 2022), with no radiotherapy or chemotherapy before surgery. All tissue samples collected were immediately stored at −80 ℃. Furthermore, 57 cases of CCA peripheral blood samples and 20 healthy cases were collected from the Laboratory Department of The Second Affiliated Hospital of Kunming Medical University (May to September 2022) for CPT2 expression verification. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments and was approved by the Ethics Committee of The Second Affiliated Hospital of Kunming Medical University (approval No. FEY-BG-39-2.0), and all patients signed informed consent forms.
Cell culture
Human CCA cell line HCCC9810 was purchased from Procell (Wuhan, China); RBE and HUCCT1 were purchased from Kunming Institute of Zoology, Chinese Academy of Sciences; human normal bile duct epithelial cells (HIBEpiC) were purchased from Otwo Biotech (Shenzhen, China). All cells were identified by the Kunming Institute of Zoology, Chinese Academy of Sciences. HCCC9810 and RBE were cultured in RPMI Medium 1640 (Gibco, NY, USA) containing 10% fetal bovine serum (FBS; Gibco, New Zealand). HIBEpiC and HUCCT1 were cultured in high glucose DMEM (Gibco, NY, USA) containing 10% FBS (Gibco, New Zealand). All cells were incubated at 5% CO2 and 37 ℃.
Cell transfection
CPT2 overexpression lentivirus and blank lentivirus vector carrying puromycin resistance and GFP were purchased from Genechem (Shanghai, China). HCCC9810 and RBE cells were transfected with overexpression lentivirus, and the stable expression cell lines were screened with 2 µg/mL puromycin after transfection for 48 h. Puromycin-resistant CPT2 knock-down lentivirus and blank scramble were purchased from Genechem. The shRNA sequences of anti-human CPT2 were sh-CPT2#1: 5'-GACCAGTGAGAACCGAGACAT-3', sh-CPT2#2: 5'-CCAGGGCTTTGACCGACACTT-3', and sh-CPT2#3: 5'-TCTCTTGAATGATGGCCAGTT-3'. After HCCC9810 cells were transfected with knockdown lentivirus for 48 h according to the manufacturer’s instructions, the stable expression cell lines were screened with 2 µg/mL puromycin. Transfection efficiency was observed under a fluorescence microscope, and overexpression and knockdown efficiency were detected by Western blotting and quantitative real-time polymerase chain reaction (qRT-PCR).
RNA extraction and qRT-PCR
Total RNA was extracted from CCA tissues, cells, and peripheral blood using Trizol reagent (TaKaRa, Japan); subsequently, RNA was reverse transcribed into cDNA using HiScript III All-in-one RT SuperMix Perfect for qPCR kit (Vazyme, Nanjing, China). Fluorescence quantitative analysis was performed using ChamQ Universal SYBR qPCR Master Mix (Vazyme, Q711-02Univer 03) to prepare the system and set machine parameters. The primer sequence was—CPT2 forward: 5'-CTTTGACCGACACTTGTTTGC-3', CPT2 reverse: 5'-ATGAACAGCATACCCAACACC-3'. glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward: 5'-GGAGCGAGATCCCTCCAAAAT-3', GAPDH reverse: 5'-GGCTGTTGTCATACTTCTCATGG-3'. Gene expression levels were calculated by the 2−ΔΔCt method (12).
Western blot
RIPA lysis buffer (Beyotime, Shanghai, China) containing protease and phosphatase inhibitor (Beyotime) was used to lyse CCA cells or tissues to obtain total proteins. Subsequently, protein concentrations were determined using a BCA protein quantitative kit (Beyotime), and sample volumes were calculated. Proteins of equal amounts were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, Ireland). The PVDF membrane was blocked with blocking solution (Beyotime) for 2 h, then washed with TBST (TBS containing 0.1% Tween 20) three times, for 5 minutes each. Following the washes, the PVDF membrane was incubated with primary antibody [CPT2, Proteintech, 26555-1-AP (Wuhan, China); GAPDH, BOSTER, A00227-1 (Wuhan, China); beta-actin, Servicebio, GB11001 (Wuhan, China); E-cadherin, Proteintech 60335-1-Ig; N-cadherin, Abclonal, A19083 (Wuhan, China); SNAI1, Proteintech, 13099-1-AP; Vimentin, Proteintech, 10366-1-AP; CDK4, Cell Signaling Technology (CST, USA), 12790; CDK6, CST, 13331; Bcl2, CST, 4223; Bax, CST, 5023; caspase-3, CST, 14220; cleaved caspase-3, CST, 9664; p65, CST, 8242; phosphorylated p65 (p-p65; Ser536) CST, 3033] at 4 ℃ overnight. Subsequently, the membrane was washed with TBST three times and then incubated with the secondary antibody (Goat anti-Rabbit, BOSTER, BA1055; Goat anti-mouse, BOSTER, BA1051) at 25 ℃ for 90 minutes. After incubation and three washes with TBST, BeyoECL Moon solution (Beyotime) was used for target protein detection.
Cell proliferation assays
Overexpressed RBE and HCCC9810 cells were inoculated into 96-well plates at 5,000 cells/well, and knockdown HCCC9810 cells were inoculated into 96-well plates at 3,000 cells/well. These cells were cultured at 37 ℃, 5% CO2, and their proliferative ability was measured by Cell Counting Kit-8 (CCK-8) kit (Meilune, Dalian, China). The cells were incubated at 37 ℃ for 2 h after adding CCK-8 solution at 0, 24, 48, 72, and 96 h. Subsequently, optical density (OD) values were measured at 450 nm.
Transwell migration and invasion assays
The upper compartment of the 8 µm chamber (Corning, USA) was evenly coated with Matrigel (BD Biosciences, USA) at a volume of 60 µL, this step was omitted for the migration assay. Cell number was adjusted to 5×104 with 200 µL serum-free medium and inoculated into the upper chamber. Subsequently, 600 µL of the corresponding medium containing 20% FBS was added to the lower chamber. After culturing at 37 ℃, 5% CO2 for 24 h, cells remaining in the upper chamber were carefully removed and fixed with 4% paraformaldehyde solution for 30 minutes. Cells were then carefully washed with phosphate buffered saline (PBS) and 1% crystal violet staining solution was added before incubation for 10 minutes. Finally, we took pictures and counted cells using a phase contrast microscope (200×).
Cell cycle and apoptosis
Cell cycle: cells were fixed overnight with 75% precooled ethanol at 4 ℃. Subsequently, cells were washed with precooled PBS, and the staining solution was prepared according to the cell cycle analysis kit (Beyotime) and added to the cells. They were incubated in the dark at 37 ℃ for 30 minutes, and then analyzed by flow cytometry.
Apoptosis: cells were washed with PBS, and binding buffer was used to adjust cell concentration to 1×106/mL; Then, 5 µL of Annexin V/PE and 5 µL of 7-AAD staining solution (BD Biosciences, USA) were then added to 100 µL of cell solution to incubate for 15 minutes in the dark. Subsequently, 400 µL of binding buffer was added, and the cells were analyzed by flow cytometry.
Statistical analysis
GraphPad Prism 7.0 software was used for statistical analysis; statistical comparisons were made between two independent groups using two-sample t-tests and between two paired groups using the paired t-test. Data are shown as mean ± standard deviation (SD). A P value <0.05 was considered statistically significant. All experiments were conducted a minimum of three times, with each experiment including at least three biological replicates.
Results
CPT2 expression was down-regulated in CCA and other cancers
GEO datasets and TCGA datasets were used to calculate the difference in CPT2 expression between cancer tissues and adjacent normal tissues. Results showed that the expression of CPT2 in cancer tissues was significantly lower than that in adjacent normal tissues in GEO datasets (Figure 1A, P<0.001), and the same conclusion was obtained in TCGA datasets (Figure 1B, P=0.02). Additionally, we conducted a pan-cancer analysis of CPT2 to understand the expression of CPT2 in various cancers. Results showed that CPT2 expression was down-regulated in 12 types of cancer (Figure 1C). To validate these results, we measured CPT2 expression in peripheral blood and fresh CCA tissues. Results showed that the mRNA level of CPT2 in peripheral blood of CCA was significantly lower than that in healthy controls (Figure 1D, P<0.001); moreover, the mRNA level and protein expression of CPT2 in cancer tissues were lower than those in adjacent normal tissues (Figure 1E,1F, P=0.003).
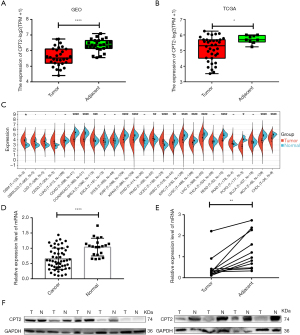
CPT2 was an independent prognostic factor for CCA and was positively correlated with CCA prognosis
To determine the independent prognostic factors of CCA, the clinicopathological features and the expression of CPT2 were analyzed by univariate and multivariate COX regression analyses. Univariate COX regression analysis revealed that CPT2 expression, vascular invasion, and clinical stage were factors affecting CCA prognosis (Figure 2A); whereas multivariate COX regression indicated that the expression of CPT2 [hazard ratio (HR), 0.065; 95% confidence interval (CI): 0.013–0.32; P<0.001] and clinical stage (HR, 8.951; 95% CI: 1.809–44.3; P=0.007) were independent prognostic factors of CCA (Figure 2B). These factors were incorporated into a nomogram to visually predict the 1-, 3-, and 5-year survival of CCA (C-index =0.8143), and a calibration curve was drawn (Figure 2C,2D). The nomogram indicated that a lower probability of CCA survival was associated with lower expression of CPT2 and higher clinical stage. The Kaplan-Meier curve showed that the survival probability with low CPT2 expression was significantly lower than that with high CPT2 expression (Figure 2E, P=0.01). Furthermore, a time-dependent ROC curve was used to evaluate the efficiency of CPT2 in predicting the 1-, 3-, and 5-year CCA survival (Figure 2F). The results showed that CPT2 had a high predictive ability for 1-year CCA survival [are under the curve (AUC) =0.933] but a low predictive ability in 3- and 5-year survival (AUC =0.61, 0.612).

The molecular mechanism of CPT2 in CCA was predicted by GSEA
GSEA was used to further predict the possible molecular mechanism of CPT2 in GEO datasets. The results showed that HALLMARK_TNFA_SIGNALING_VIA_NFKB (Figure 3A), HALLMARK_HYPOXIA (Figure 3B), and HALLMARK_EPITHELIAL_MESENCHYMAL_TRANSITION (Figure 3C) gene sets were significantly enriched in the low-CPT2-expression group. This suggested that CPT2 may act as a tumor suppressor gene in CCA by inhibiting TNFα/NF-κB, hypoxia, and epithelial-mesenchymal transition (EMT) signaling pathways. Furthermore, correlations between the expression of CPT2 and NF-κB transcription factor suppressor genes (NFKBIA, NFKBIB) were analyzed using the cBioPortal database (Figure 3D,3E). Results showed that CPT2 expression was positively correlated with NFKBIA expression (P=0.050), and also positively correlated with NFKBIB expression, but the difference was not statistically significant (P=0.06). Simultaneously, we analyzed the correlation between CPT2 expression and phosphorylated (S536 site) protein (NFKB1_PS536), a key protein that is required for the activation of the NF-κB pathway (Figure 3F, P=0.01). We found that CPT2 expression was negatively correlated with NFKB1_PS536 expression, suggesting that a low expression of CPT2 in CCA may be closely related to the activation of the NF-κB pathway and vice versa. Finally, we analyzed the correlation between the expression of CPT2 and CDH2 (Figure 3G), which showed that CPT2 expression was negatively correlated with CDH2 expression, though the difference was not statistically significant (P=0.06). This suggested that a low expression of CPT2 may activate the EMT pathway and cause malignant metastasis of tumor cells in CCA.

Overexpression of CPT2 inhibited the proliferation of CCA cells
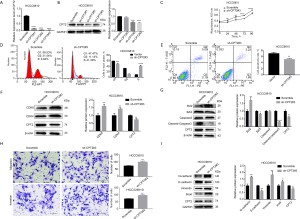
To further understand the effect of CPT2 on the biological function of CCA cells, we quantified the expression of CPT2 in CCA cells (HCCC9810, RBE, and HUCCT1) and HIBEpiC using qRT-PCR and Western blot. Results showed that mRNA levels and protein expressions of CPT2 in CCA cells were lower than those in HIBEpiC (Figure 4A). Subsequently, given that RBE and HCCC9810 cells demonstrate greater proliferation and invasiveness compared to HUCCT1 cells, they are better suited to simulate the biological behavior of CCA. We overexpressed CPT2 in HCCC9810 and RBE cells. The results of fluorescence density (Figure 4B), qRT-PCR, and Western blotting indicated that CPT2 was successfully overexpressed (Figure 4C,4D). In addition, we explored the effect of overexpression of CPT2 on the proliferation of CCA cells by CCK-8. Results showed that the proliferation ability of the overexpressed CPT2 group was significantly lower than that of the vector group (Figure 4E), suggesting that CPT2 could inhibit CCA cell proliferation.

Overexpression of CPT2 arrested CCA cells in G1 phase and induced apoptosis
Following the observation that CPT2 could inhibit the proliferation of CCA cells, we further analyzed the cell cycle and apoptosis of CCA cells using flow cytometry and then analyzed the expression of cycle-related proteins (CDK4, CDK6) and apoptosis-related proteins (Bcl2, Bax, caspase-3, and cleaved-casepase-3) using Western blotting. Results indicated that the proportion of cells in the G1 phase was higher in the overexpressed CPT2 group compared to the control group, while S phase was decreased (Figure 5A,5B). Apoptosis results revealed that the apoptotic proportion in the overexpressed CPT2 group was higher than that in the control group (Figure 5C,5D), Additionally, the expression of CDK4 and CDK6 in the overexpressed CPT2 group was also down-regulated (Figure 5E), indicating that CPT2 may inhibit the proliferation of CCA cells by blocking their progression from G1 to S phase. And the expression of Bax and cleaved-caspase-3 in the overexpressed CPT2 group was increased, while the expression of Bcl2 was decreased (Figure 5F), indicating that CPT2 could induce apoptosis in CCA cells.

Overexpression of CPT2 inhibited migration and invasion of CCA cells by inhibiting EMT
We investigated the impact of CPT2 overexpression on the invasion and migration of CCA cells using the transwell assay and analyzed the expression of EMT-related proteins using Western blotting. Results indicated that the migration and invasive abilities of the CPT2-overexpressing group were weaker than those of the vector group (Figure 6A,6B), and the expression of E-cadherin was higher, while the expressions of N-cadherin, vimentin and snail protein were lower in the CPT2-overexpressing group (Figure 6C). This indicates that CPT2 could inhibit the invasion and migration of CCA cells, exerting an inhibitory effect on the EMT signaling pathway.

CPT2 knockdown could promote the proliferation, migration, and invasion of CCA cells
To further verify the effect of CPT2 on the biological function of CCA cells, we knocked down CPT2 in HCCC9810 cells. The results of qRT-PCR and Western blotting showed that CPT2 was knocked down successfully in HCCC9810 cells, with the highest knockdown efficiency observed for sh-CPT2#3 (Figure 7A,7B). CCK-8 results indicated that CPT2 knockdown could enhance the cell viability of HCCC9810 (Figure 7C). Flow cytometry showed that compared to the Scramble group, the proportion of cells in the G1 phase was lower, while the S phase was higher (Figure 7D), and the proportion of apoptosis was lower in the CPT2 knockdown group (Figure 7E). Western blotting showed that CPT2 knockdown could up-regulate the expression of CDK4, CDK6 and Bcl2, while down-regulating the expression of Bax and cleaved caspase-3 (Figure 7F,7G), indicating that CPT2 knockdown could promote more HCCC9810 cells to transition from G1 to S phase, thereby enhancing proliferation and inhibiting apoptosis. Additionally, transwell assays demonstrated that CPT2 knockdown could significantly enhance the migration and invasion ability of HCCC9810 cells (Figure 7H); Western blotting showed that this effect was mediated by activation of the EMT pathway (Figure 7I).
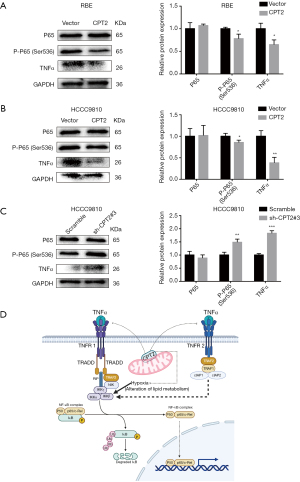
The inhibitory effect of CPT2 on CCA cells may be related to the inhibition of the TNFα/NF-κB signal pathway.
Combined with the results of GSEA, we further verified the relationship between CPT2 and the TNFα/NF-κB signaling pathway using Western blotting. Results indicated that the expressions of TNFα and p-p65 were down-regulated in the overexpressed CPT2 groups (Figure 8A,8B), while knockdown of CPT2 gave the opposite results (Figure 8C). In addition, we mapped the specific biological process of CPT2 regulating the TNFα/NF-κB signaling pathway (Figure 8D). The figure demonstrates that CPT2 down-regulates the expression of TNFα, thereby reducing the binding of TNFα to TNFR1 and TNFR2, and blocking the activation of the NF-κB pathway.
Discussion
CCA is the second most common primary liver cancer with a high degree of malignancy. Unfortunately, the recurrence rate after CCA resection is high, the prognosis is poor, and the 5-year survival rate is only 50–70% (13). It is therefore important to explore potential therapeutic targets and identify accurate prognostic markers. Numerous studies have shown that the expression of CPT2 is down-regulated in hepatocellular carcinoma (9), colorectal cancer (10,14), and ovarian cancer (11), promoting tumor progression through various pathways and is closely related to poor prognosis. Furthermore, a previous study has shown that CPT2 could be used as an independent prognostic factor for colorectal cancer (15). CPT2 is closely related to tumor development and is a potential therapeutic target and prognostic marker, but its role in CCA is unclear.
In this study, we identified the low expression of CPT2 in CCA from GEO and TCGA datasets; CPT2 and clinical stage can serve as independent prognostic factors for CCA. CPT2 effectively predicted the 1-, 3-, and 5-year survival of CCA patients, indicating its potential as a prognostic marker for CCA. Furthermore, we verified the low expression of CPT2 in CCA tissues and cells and confirmed its inhibitory effect on the proliferation, migration, and invasion of CCA cells. This suggests that CPT2 can inhibit the malignant progression of CCA and may be a potential therapeutic target.
While exploring the mechanism of CPT2 inhibition of proliferative CCA cells, we observed no significant difference between the overexpression group and the vector group in RBE cells at 96 hours. This could be attributed to variations in experimental conditions at different time points, such as differences in culture medium composition, cell density, and culture environment, which can influence cell proliferation. During the 96-hour growth period, the cell density in the well plates of both the overexpression and vector groups may have reached a level that triggered contact inhibition, preventing further cell proliferation and resulting in similar absorbance values between the groups. Additionally, the consumption of nutrients and the accumulation of metabolites in the culture medium may vary at different time points, thereby affecting the cell response to CPT2 overexpression. As the culture time extends, RBE cells may counteract some of the inhibitory effects of CPT2 overexpression by modulating their metabolic and signaling pathways. For example, cells might compensate for the proliferation inhibition caused by CPT2 overexpression by enhancing the activity of alternative proliferation-related signaling pathways. Considering the potential impact of these factors, we subsequently conducted cell cycle experiments and analyzed proliferation-related proteins to comprehensively evaluate the effects of CPT2 overexpression on the proliferation capacity of RBE cells, with the goal of providing a more accurate explanation of this contradictory phenomenon. we found that CPT2 could arrest CCA cells in the G1 phase, decrease the proportion of S-phase cells, and induce apoptosis. Additionally, our study revealed that CPT2 could down-regulate the expression of CDK4/CDK6. Previous studies have revealed that CDK4/6-CyclinD complexes could phosphorylate members of the retinoblastoma (RB) protein family and release E2F transcription factors, thereby activating the E2F-dependent genes, enabling cells to transition from the G1 phase to the S phase and synthesize DNA. This suggests that CPT2 can inhibit the transition of CCA cells from the G1 phase to the S phase and weaken the proliferative ability of CCA cells through down-regulating CDK4/CDK6 expression. Therefore, inhibiting CDK4/CDK6 expression is an effective method for treating tumors; many studies have shown that CDK4/CDK6 inhibitors play an important anticancer role in CCA (16,17), gastric cancer (18), esophageal squamous cell carcinoma (19), colorectal cancer (20), and breast cancer (21). Moreover, this study showed that CPT2 inhibited the expression of Bcl2 while up-regulating the expression of Bax and cleaved-caspase-3, which promoted apoptosis and inhibited CCA cell growth. These results suggest that CPT2 may be a tumor suppressor gene in CCA, and up-regulating the expression of CPT2 may be a new strategy for the treatment of CCA.
EMT refers to the process in which epithelial cells lose their junctions and basal-apical polarity and acquire the ability of mesenchymal cells to migrate and invade surrounding tissues (22). Therefore, EMT is an important means through which tumor cells acquire capacity for invasion and metastasis. Many studies show that EMT is frequently activated in CCA and promotes the migration and invasion of CCA cells (23,24). Therefore, inhibition of the EMT pathway is a reliable method for inhibiting the malignant progression of CCA. Here, we demonstrated that CPT2 inhibited the migration and invasion of CCA cells by inhibiting EMT. This suggests that CPT2 may be a key factor in inhibiting malignant metastasis of CCA.
In terms of mechanism, previous studies have shown that down-regulation of CPT2 expression can promote tumor growth by increasing the expression of SCD1 (9), or via the ROS/Wnt/β-catenin (14), ROS/NF-κB (11), and p53 (10) signaling pathways. Here, we found that hypoxia and the TNFα/NF-κB signaling pathways were significantly enriched in the group with low expression of CPT2 in CCA. Many studies have demonstrated that hypoxia and the TNFα/NF-κB signaling pathways are frequently activated in cancers to promote the malignant phenotype of tumor cells (25,26). Hypoxia significantly contributes to the pathogenesis of CCA. Under hypoxic conditions, CCA cells exhibit heightened glycogen phosphorylase activity, which drives metabolic reprogramming. This shift promotes glycolysis, thereby supporting tumorigenesis (27). Furthermore, hypoxia enhances fatty acid synthesis, which not only promotes CCA progression but also contributes to chemotherapy resistance (28). Moreover, hypoxia has been shown to downregulate the expression of the CPT2 gene (29), specifically affecting lysine 457/8 in CPT2, leading to enzyme inactivation (30). This indicates that CPT2 expression may be down-regulated during the malignant progression of CCA, leading to the activation of hypoxia and the TNFα/NF-κB signaling pathways. For the specific biological process of the hypoxia signaling pathway regulated by CPT2, we hypothesize that during CCA malignancy, the expression of CPT2 is down-regulated, leading to alterations in fatty acid oxidation and amino acid metabolism. These disturbances lead to hypoxia, high acidity, and nutritional deficiency of the tumor microenvironment (TME), which is conducive to tumor growth (31). Thus, the hypoxia pathway becomes activated. For the TNFα/NF-κB signaling pathway, we analyzed the expression of TNFα, p65, and p-p65 (Ser536) in the overexpression and knockdown groups. Results indicated that TNFα and p-p65 expression were down-regulated after CPT2 overexpression, but the opposite results were found after CPT2 knockdown. This indicates that downregulation of CPT2 in CCA relieves its inhibitory effect on TNFα expression. TNFα binds to TNFR1 or TNFR2 more frequently, and the downstream NF-κB signaling pathway is activated. Furthermore, hypoxia caused by changes in lipid metabolism can activate the NF-κB signaling pathway (32,33) (Figure 8D). However, further research is necessary to confirm this hypothesis, as it could have significant implications for understanding the molecular mechanisms underlying CCA progression and resistance to therapy.
In summary, CPT2 expression is downregulated in CCA and can serve as a potential prognostic marker for CCA patients. However, further verification is needed. If CPT2 is to be used as an indicator in routine biopsies for CCA, immunohistochemistry (IHC) detection of CPT2 expression is crucial. This is a limitation in the manuscript and needs to be improved in the future studies. Moreover, CPT2 functions as a tumor suppressor gene primarily by inhibiting the TNFα/NF-κB signaling pathway in CCA, making it a promising target for CCA treatment. Furthermore, further study is needed to determine whether the effects of CPT2 in CCA are consistent between in vivo and in vitro settings.
Conclusions
This study presents compelling evidence that CPT2, a key enzyme in fatty acid oxidation, is downregulated in CCA and serves as a potential prognostic biomarker. Our findings indicate that CPT2 expression is inversely correlated with the progression of CCA, with lower expression levels associated with poor patient prognosis. Through bioinformatics analysis, Cox regression, and nomogram prediction models, we have demonstrated that CPT2 expression and clinical stage are independent prognostic factors for CCA. Functional assays further clarify the tumor-suppressive role of CPT2 in CCA, as its overexpression inhibits cell proliferation, induces cell cycle arrest at the G1 phase, promotes apoptosis, and represses EMT, thereby diminishing cell migration and invasion. Mechanistically, our data indicate that CPT2 exerts its inhibitory effects on CCA cells by modulating the TNFα/NF-κB signaling pathway, suggesting a novel therapeutic target for CCA intervention. Collectively, our results underscore the significance of CPT2 as a prognostic marker and its potential utility as a therapeutic target in the management of CCA, warranting further investigation into its clinical applications.
Acknowledgments
The authors would like to express their gratitude to Dr. Yifan Bao (Group of Analytical Food Chemistry and Lipid Oxidation, Department of Physiological Chemistry, University of Vienna) for her help and support in creating Figure 8D.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-685/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-685/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-685/prf
Funding: The current study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-685/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments and was approved by the Ethics Committee of The Second Affiliated Hospital of Kunming Medical University (approval No. FEY-BG-39-2.0), and all patients signed informed consent forms.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dai YS, Hu HJ, Lv TR, et al. The influence of resection margin width in patients with intrahepatic cholangiocarcinoma: a meta-analysis. World J Surg Oncol 2023;21:16. [Crossref] [PubMed]
- Krasinskas AM. Cholangiocarcinoma. Surg Pathol Clin 2018;11:403-29. [Crossref] [PubMed]
- Stüben BO, Ahmadi S, Saner FH, et al. The significance of resection margins on R0 results in intrahepatic cholangiocarcinoma. Surg Oncol 2024;53:102058. [Crossref] [PubMed]
- Gupta A, Sahai P, Prasad M, et al. Treatment Response and Survival with Chemotherapy for Unresectable, Nonmetastatic Cholangiocarcinoma. Euroasian J Hepatogastroenterol 2024;14:5-8. [Crossref] [PubMed]
- Zhang Y, Esmail A, Mazzaferro V, et al. Newest Therapies for Cholangiocarcinoma: An Updated Overview of Approved Treatments with Transplant Oncology Vision. Cancers (Basel) 2022;14:5074. [Crossref] [PubMed]
- Cheng H, Wang M, Su J, et al. Lipid Metabolism and Cancer. Life (Basel) 2022;12:784. [Crossref] [PubMed]
- Guo R, Chen Y, Borgard H, et al. The Function and Mechanism of Lipid Molecules and Their Roles in The Diagnosis and Prognosis of Breast Cancer. Molecules 2020;25:4864. [Crossref] [PubMed]
- Lee H, Woo SM, Jang H, et al. Cancer depends on fatty acids for ATP production: A possible link between cancer and obesity. Semin Cancer Biol 2022;86:347-57. [Crossref] [PubMed]
- Lin M, Lv D, Zheng Y, et al. Downregulation of CPT2 promotes tumorigenesis and chemoresistance to cisplatin in hepatocellular carcinoma. Onco Targets Ther 2018;11:3101-10. [Crossref] [PubMed]
- Liu F, Li X, Yan H, et al. Downregulation of CPT2 promotes proliferation and inhibits apoptosis through p53 pathway in colorectal cancer. Cell Signal 2022;92:110267. [Crossref] [PubMed]
- Zhang X, Zhang Z, Liu S, et al. CPT2 down-regulation promotes tumor growth and metastasis through inducing ROS/NFκB pathway in ovarian cancer. Transl Oncol 2021;14:101023. [Crossref] [PubMed]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402-8. [Crossref] [PubMed]
- Buckholz AP, Brown RS Jr. Cholangiocarcinoma: Diagnosis and Management. Clin Liver Dis 2020;24:421-36. [Crossref] [PubMed]
- Li H, Chen J, Liu J, et al. CPT2 downregulation triggers stemness and oxaliplatin resistance in colorectal cancer via activating the ROS/Wnt/β-catenin-induced glycolytic metabolism. Exp Cell Res 2021;409:112892. [Crossref] [PubMed]
- Liu J, Li Y, Xiao Q, et al. Identification of CPT2 as a prognostic biomarker by integrating the metabolism-associated gene signature in colorectal cancer. BMC Cancer 2022;22:1038. [Crossref] [PubMed]
- Suppramote O, Prasopporn S, Aroonpruksakul S, et al. The Acquired Vulnerability Caused by CDK4/6 Inhibition Promotes Drug Synergism Between Oxaliplatin and Palbociclib in Cholangiocarcinoma. Front Oncol 2022;12:877194. [Crossref] [PubMed]
- Goel S, Bergholz JS, Zhao JJ. Targeting CDK4 and CDK6 in cancer. Nat Rev Cancer 2022;22:356-72. [Crossref] [PubMed]
- Liu Y, Zhao Y, Han C, et al. Expression of CDK6 in Stomach Cancer and the Effect of CDK4/6 Inhibitor PD-0332991 on the Function of Stomach Cancer Cells. Comput Math Methods Med 2022;2022:2402567. Retracted Publication. [Crossref] [PubMed]
- Jubashi A, Kotani D, Kojima T, et al. Current landscape of targeted therapy in esophageal squamous cell carcinoma. Curr Probl Cancer 2024;53:101152. [Crossref] [PubMed]
- Thoma OM, Neurath MF, Waldner MJ. Cyclin-Dependent Kinase Inhibitors and Their Therapeutic Potential in Colorectal Cancer Treatment. Front Pharmacol 2021;12:757120. [Crossref] [PubMed]
- Wang X, Zhao S, Xin Q, et al. Recent progress of CDK4/6 inhibitors' current practice in breast cancer. Cancer Gene Ther 2024;31:1283-91. [Crossref] [PubMed]
- Manfioletti G, Fedele M. Epithelial-Mesenchymal Transition (EMT) 2021. Int J Mol Sci 2022;23:5848. [Crossref] [PubMed]
- Liang S, Guo H, Ma K, et al. A PLCB1-PI3K-AKT Signaling Axis Activates EMT to Promote Cholangiocarcinoma Progression. Cancer Res 2021;81:5889-903. [Crossref] [PubMed]
- Chen Z, Li H, Li Z, et al. SHH/GLI2-TGF-β1 feedback loop between cancer cells and tumor-associated macrophages maintains epithelial-mesenchymal transition and endoplasmic reticulum homeostasis in cholangiocarcinoma. Pharmacol Res 2023;187:106564. [Crossref] [PubMed]
- Chen J, Qiao K, Zhang C, et al. VRK2 activates TNFα/NF-κB signaling by phosphorylating IKKβ in pancreatic cancer. Int J Biol Sci 2022;18:1288-302. [Crossref] [PubMed]
- Hapke RY, Haake SM. Hypoxia-induced epithelial to mesenchymal transition in cancer. Cancer Lett 2020;487:10-20. [Crossref] [PubMed]
- Pan Y, Zhou Y, Shen Y, et al. Hypoxia Stimulates PYGB Enzymatic Activity to Promote Glycogen Metabolism and Cholangiocarcinoma Progression. Cancer Res 2024;84:3803-17. [Crossref] [PubMed]
- Chen Y, Xu X, Wang Y, et al. Hypoxia-induced SKA3 promoted cholangiocarcinoma progression and chemoresistance by enhancing fatty acid synthesis via the regulation of PAR-dependent HIF-1a deubiquitylation. J Exp Clin Cancer Res 2023;42:265. [Crossref] [PubMed]
- Chen J, Chen J, Fu H, et al. Hypoxia exacerbates nonalcoholic fatty liver disease via the HIF-2α/PPARα pathway. Am J Physiol Endocrinol Metab 2019;317:E710-22. [Crossref] [PubMed]
- Mao Y, Zhang J, Zhou Q, et al. Hypoxia induces mitochondrial protein lactylation to limit oxidative phosphorylation. Cell Res 2024;34:13-30. [Crossref] [PubMed]
- Lian X, Yang K, Li R, et al. Immunometabolic rewiring in tumorigenesis and anti-tumor immunotherapy. Mol Cancer 2022;21:27. [Crossref] [PubMed]
- Gu D, Ye M, Zhu G, et al. Hypoxia upregulating ACSS2 enhances lipid metabolism reprogramming through HMGCS1 mediated PI3K/AKT/mTOR pathway to promote the progression of pancreatic neuroendocrine neoplasms. J Transl Med 2024;22:93. [Crossref] [PubMed]
- Si J, Guo J, Zhang X, et al. Hypoxia-induced activation of HIF-1alpha/IL-1beta axis in microglia promotes glioma progression via NF-κB-mediated upregulation of heparanase expression. Biol Direct 2024;19:45. [Crossref] [PubMed]


